Progress towards Adjuvant Development: Focus on Antiviral Therapy
- PMID: 37298177
- PMCID: PMC10253057
- DOI: 10.3390/ijms24119225
Progress towards Adjuvant Development: Focus on Antiviral Therapy
Abstract
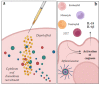
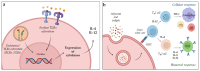
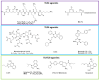
In recent decades, vaccines have been extraordinary resources to prevent pathogen diffusion and cancer. Even if they can be formed by a single antigen, the addition of one or more adjuvants represents the key to enhance the response of the immune signal to the antigen, thus accelerating and increasing the duration and the potency of the protective effect. Their use is of particular importance for vulnerable populations, such as the elderly or immunocompromised people. Despite their importance, only in the last forty years has the search for novel adjuvants increased, with the discovery of novel classes of immune potentiators and immunomodulators. Due to the complexity of the cascades involved in immune signal activation, their mechanism of action remains poorly understood, even if significant discovery has been recently made thanks to recombinant technology and metabolomics. This review focuses on the classes of adjuvants under research, recent mechanism of action studies, as well as nanodelivery systems and novel classes of adjuvants that can be chemically manipulated to create novel small molecule adjuvants.
Keywords: formulations; immune modulators; immune potentiators; small molecules; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wu Z., Liu K. Overview of vaccine adjuvants. Med. Drug Discov. 2021;11:100103. doi: 10.1016/j.medidd.2021.100103. - DOI
-
- Morel S., Didierlaurent A., Bourguignon P., Delhaye S., Baras B., Jacob V., Planty C., Elouahabi A., Harvengt P., Carlsen H., et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. - DOI - PubMed
-
- Detienne S., Welsby I., Collignon C., Wouters S., Coccia M., Delhaye S., Van Maele L., Thomas S., Swertvaegher M., Detavernier A., et al. Central Role of CD169+ Lymph Node Resident Macrophages in the Adjuvanticity of the QS-21 Component of AS01. Sci. Rep. 2016;6:39475. doi: 10.1038/srep39475. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

