Recent Developments in the Synthesis of HIV-1 Integrase Strand Transfer Inhibitors Incorporating Pyridine Moiety
- PMID: 37298265
- PMCID: PMC10253443
- DOI: 10.3390/ijms24119314
Recent Developments in the Synthesis of HIV-1 Integrase Strand Transfer Inhibitors Incorporating Pyridine Moiety
Abstract
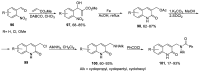
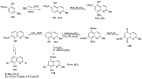
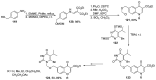
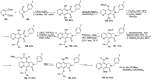
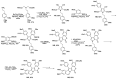
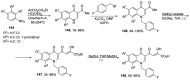
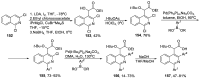
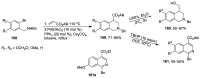
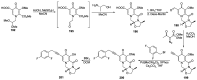
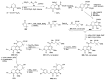
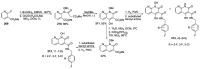
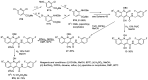
Human immunodeficiency virus (HIV) causes one of the most dangerous diseases-acquired immunodeficiency syndrome (AIDS). An estimated about 40 million people are currently living with HIV worldwide, most of whom are already on antiretroviral therapy. This makes the development of effective drugs to combat this virus very relevant. Currently, one of the dynamically developing areas of organic and medicinal chemistry is the synthesis and identification of new compounds capable of inhibiting HIV-1 integrase-one of the HIV enzymes. A significant number of studies on this topic are published annually. Many compounds inhibiting integrase incorporate pyridine core. Therefore, this review is an analysis of the literature on the methods for the synthesis of pyridine-containing HIV-1 integrase inhibitors since 2003 to the present.
Keywords: HIV-1 integrase; human immunodeficiency virus; inhibitors; pyridine; synthetic pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











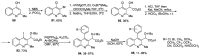
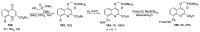
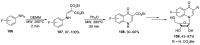

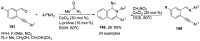





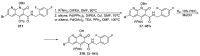
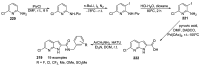


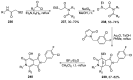
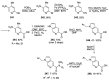




Similar articles
-
HIV-1 integrase tetramers are the antiviral target of pyridine-based allosteric integrase inhibitors.Elife. 2019 May 23;8:e46344. doi: 10.7554/eLife.46344. Elife. 2019. PMID: 31120420 Free PMC article.
-
Review of integrase strand transfer inhibitors for the treatment of human immunodeficiency virus infection.Expert Rev Anti Infect Ther. 2015;13(10):1195-212. doi: 10.1586/14787210.2015.1075393. Epub 2015 Aug 9. Expert Rev Anti Infect Ther. 2015. PMID: 26293294 Review.
-
Integrase Strand Transfer Inhibitors Are Effective Anti-HIV Drugs.Viruses. 2021 Jan 29;13(2):205. doi: 10.3390/v13020205. Viruses. 2021. PMID: 33572956 Free PMC article. Review.
-
New developments in diketo-containing inhibitors of HIV-1 integrase.Mini Rev Med Chem. 2007 Jul;7(7):707-25. doi: 10.2174/138955707781024535. Mini Rev Med Chem. 2007. PMID: 17627583 Review.
-
Design, synthesis, and biological evaluation of novel tricyclic HIV-1 integrase inhibitors by modification of its pyridine ring.Bioorg Med Chem Lett. 2006 Aug 1;16(15):3985-8. doi: 10.1016/j.bmcl.2006.05.018. Epub 2006 May 24. Bioorg Med Chem Lett. 2006. PMID: 16723226
Cited by
-
Special Issue "Development and Synthesis of Biologically Active Compounds".Int J Mol Sci. 2024 Apr 4;25(7):4015. doi: 10.3390/ijms25074015. Int J Mol Sci. 2024. PMID: 38612824 Free PMC article.
-
Chalcone derivatives containing 1,2,4-triazole and pyridine moiety: design, synthesis, and antiviral activity.Mol Divers. 2024 Dec 2. doi: 10.1007/s11030-024-11049-7. Online ahead of print. Mol Divers. 2024. PMID: 39617870
-
Nitropyridines in the Synthesis of Bioactive Molecules.Pharmaceuticals (Basel). 2025 May 7;18(5):692. doi: 10.3390/ph18050692. Pharmaceuticals (Basel). 2025. PMID: 40430510 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

