Impact of Solute Carrier Transporters in Glioma Pathology: A Comprehensive Review
- PMID: 37298344
- PMCID: PMC10253701
- DOI: 10.3390/ijms24119393
Impact of Solute Carrier Transporters in Glioma Pathology: A Comprehensive Review
Abstract
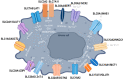
Solute carriers (SLCs) are essential for brain physiology and homeostasis due to their role in transporting necessary substances across cell membranes. There is an increasing need to further unravel their pathophysiological implications since they have been proposed to play a pivotal role in brain tumor development, progression, and the formation of the tumor microenvironment (TME) through the upregulation and downregulation of various amino acid transporters. Due to their implication in malignancy and tumor progression, SLCs are currently positioned at the center of novel pharmacological targeting strategies and drug development. In this review, we discuss the key structural and functional characteristics of the main SLC family members involved in glioma pathogenesis, along with their potential targeting options to provide new opportunities for CNS drug design and more effective glioma management.
Keywords: SLC; brain tumors; gliomas; solute carriers; therapy; transporters; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Dvorak V., Wiedmer T., Ingles-Prieto A., Altermatt P., Batoulis H., Bärenz F., Bender E., Digles D., Dürrenberger F., Heitman L.H., et al. An Overview of Cell-Based Assay Platforms for the Solute Carrier Family of Transporters. Front. Pharmacol. 2021;12:722889. doi: 10.3389/fphar.2021.722889. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

