TRAX-CHEMxt: Towards the Homogeneous Chemical Stage of Radiation Damage
- PMID: 37298351
- PMCID: PMC10253526
- DOI: 10.3390/ijms24119398
TRAX-CHEMxt: Towards the Homogeneous Chemical Stage of Radiation Damage
Abstract
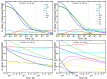
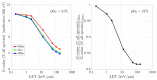
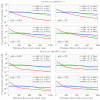
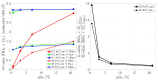
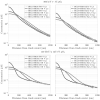
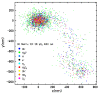
The indirect effect of radiation plays an important role in radio-induced biological damages. Monte Carlo codes have been widely used in recent years to study the chemical evolution of particle tracks. However, due to the large computational efforts required, their applicability is typically limited to simulations in pure water targets and to temporal scales up to the µs. In this work, a new extension of TRAX-CHEM is presented, namely TRAX-CHEMxt, able to predict the chemical yields at longer times, with the capability of exploring the homogeneous biochemical stage. Based on the species coordinates produced around one track, the set of reaction-diffusion equations is solved numerically with a computationally light approach based on concentration distributions. In the overlapping time scale (500 ns-1 µs), a very good agreement to standard TRAX-CHEM is found, with deviations below 6% for different beam qualities and oxygenations. Moreover, an improvement in the computational speed by more than three orders of magnitude is achieved. The results of this work are also compared with those from another Monte Carlo-based algorithm and a fully homogeneous code (Kinetiscope). TRAX-CHEMxt will allow for studying the variation in chemical endpoints at longer timescales with the introduction, as the next step, of biomolecules, for more realistic assessments of biological response under different radiation and environmental conditions.
Keywords: chemical track structure; homogeneous biochemical stage; radical/molecule yields; reaction–diffusion equations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Scifoni E. Radiation biophysical aspects of charged particles: From the nanoscale to therapy. Mod. Phys. Lett. A. 2015;30:1540019. doi: 10.1142/S0217732315400192. - DOI
-
- Meesat R., Sanguanmith S., Meesungnoen J., Lepage M., Khalil A., Jay-Gerin J.P. Utilization of the ferrous sulfate (Fricke) dosimeter for evaluating the radioprotective potential of cystamine: Experiment and Monte Carlo simulation. Radiat. Res. 2012;177:813–826. doi: 10.1667/RR2829.1. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

