The Product of the Fission Yeast fhl1 Gene Binds to the HomolE Box and Activates In Vitro Transcription of Ribosomal Protein Genes
- PMID: 37298423
- PMCID: PMC10253590
- DOI: 10.3390/ijms24119472
The Product of the Fission Yeast fhl1 Gene Binds to the HomolE Box and Activates In Vitro Transcription of Ribosomal Protein Genes
Abstract
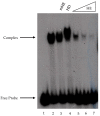
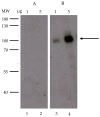
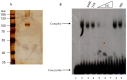
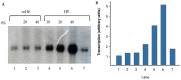
Fission yeast ribosomal protein genes (RPGs) contain a HomolD box as a core promoter element required for transcription. Some of the RPGs also contain a consensus sequence named HomolE, located upstream of the HomolD box. The HomolE box acts as an upstream activating sequence (UAS), and it is able to activate transcription in RPG promoters containing a HomolD box. In this work, we identified a HomolE-binding protein (HEBP) as a polypeptide of 100 kDa, which was able to bind to the HomolE box in a Southwestern blot assay. The features of this polypeptide were similar to the product of the fhl1 gene of fission yeast. The Fhl1 protein is the homolog of the FHL1 protein of budding yeast and possesses fork-head-associated (FHA) and fork-head (FH) domains. The product of the fhl1 gene was expressed and purified from bacteria, and it was demonstrated that is able to bind the HomolE box in an electrophoretic mobility assay (EMSA), as well as being able to activate in vitro transcription from an RPG gene promoter containing HomolE boxes upstream of the HomolD box. These results indicate that the product of the fhl1 gene of fission yeast can bind to the HomolE box, and it activates the transcription of RPGs.
Keywords: Fhl1 protein; HomolD box; HomolE box; RPGs transcription.
Conflict of interest statement
The authors declare no conflict of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
RNA polymerase II components and Rrn7 form a preinitiation complex on the HomolD box to promote ribosomal protein gene expression in Schizosaccharomyces pombe.FEBS J. 2017 Feb;284(4):615-633. doi: 10.1111/febs.14006. Epub 2017 Feb 5. FEBS J. 2017. PMID: 28060464
-
Rrn7 protein, an RNA polymerase I transcription factor, is required for RNA polymerase II-dependent transcription directed by core promoters with a HomolD box sequence.J Biol Chem. 2011 Jul 29;286(30):26480-6. doi: 10.1074/jbc.M111.224337. Epub 2011 Jun 14. J Biol Chem. 2011. PMID: 21673110 Free PMC article.
-
E2F site activates transcription in fission yeast Schizosaccharomyces pombe and binds to a 30-kDa transcription factor.J Biol Chem. 1993 Sep 25;268(27):20392-401. J Biol Chem. 1993. PMID: 8376397
-
Physiological function of FKBP12, a primary target of rapamycin/FK506: a newly identified role in transcription of ribosomal protein genes in yeast.Curr Genet. 2021 Jun;67(3):383-388. doi: 10.1007/s00294-020-01142-3. Epub 2021 Jan 12. Curr Genet. 2021. PMID: 33438053 Review.
-
Transcriptional control of ribosome biogenesis in yeast: links to growth and stress signals.Biochem Soc Trans. 2021 Aug 27;49(4):1589-1599. doi: 10.1042/BST20201136. Biochem Soc Trans. 2021. PMID: 34240738 Free PMC article. Review.
References
-
- Bielka H., Stahl J. Structure and function of eukaryotic ribosomes [Protein biosynthesis, plants, animals] Int. Rev. Biochem. 1978;18:79–168.
-
- Witt I., Kivinen K., Käufer N.F. The Molecular Biology of Schizosaccharomyces Pombe. Springer; Berlin/Heidelberg, Germany: 2004. Core Promoters in S. pombe: TATA and HomolD boxes; pp. 343–351. Genetics, Genomics and Beyond. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

