Chondrocyte Thrombomodulin Protects against Osteoarthritis
- PMID: 37298473
- PMCID: PMC10253941
- DOI: 10.3390/ijms24119522
Chondrocyte Thrombomodulin Protects against Osteoarthritis
Abstract
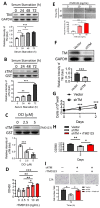
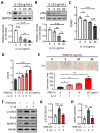
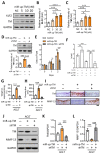
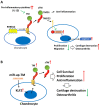
Osteoarthritis (OA) is a prevalent form of arthritis that affects over 32.5 million adults worldwide, causing significant cartilage damage and disability. Unfortunately, there are currently no effective treatments for OA, highlighting the need for novel therapeutic approaches. Thrombomodulin (TM), a glycoprotein expressed by chondrocytes and other cell types, has an unknown role in OA. Here, we investigated the function of TM in chondrocytes and OA using various methods, including recombinant TM (rTM), transgenic mice lacking the TM lectin-like domain (TMLeD/LeD), and a microRNA (miRNA) antagomir that increased TM expression. Results showed that chondrocyte-expressed TM and soluble TM [sTM, like recombinant TM domain 1 to 3 (rTMD123)] enhanced cell growth and migration, blocked interleukin-1β (IL-1β)-mediated signaling and protected against knee function and bone integrity loss in an anterior cruciate ligament transection (ACLT)-induced mouse model of OA. Conversely, TMLeD/LeD mice exhibited accelerated knee function loss, while treatment with rTMD123 protected against cartilage loss even one-week post-surgery. The administration of an miRNA antagomir (miR-up-TM) also increased TM expression and protected against cartilage damage in the OA model. These findings suggested that chondrocyte TM plays a crucial role in counteracting OA, and miR-up-TM may represent a promising therapeutic approach to protect against cartilage-related disorders.
Keywords: miRNA; osteoarthritis; thrombomodulin; transgenic mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 110-2314-B-037-022-/Ministry of Science and Technology, Executive Yuan, Taiwan
- 111-2314-B-037-055-/Ministry of Science and Technology, Executive Yuan, Taiwan
- 110-2314-B-006 -037 -MY3/Ministry of Science and Technology, Executive Yuan, Taiwan
- 110-2314-B-037 -029 -MY3/Ministry of Science and Technology, Executive Yuan, Taiwan
- 111-2314-B-037-106/Ministry of Science and Technology, Executive Yuan, Taiwan
LinkOut - more resources
Full Text Sources
Medical

