An Update on Familial Mediterranean Fever
- PMID: 37298536
- PMCID: PMC10253709
- DOI: 10.3390/ijms24119584
An Update on Familial Mediterranean Fever
Abstract
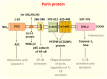
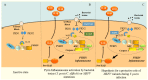
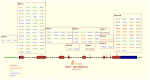
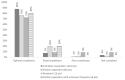
(1) Background: Familial Mediterranean Fever (FMF) is the prototypal autoinflammatory disease, characterized by recurrent bursts of neutrophilic inflammation. (2) Methods: In this study we look at the most recent literature on this condition and integrate it with novel information on treatment resistance and compliance. (3) Results: The canonical clinical presentation of FMF is in children with self-limited episodes of fever and polyserositis, associated with severe long-term complications, such as renal amyloidosis. It has been described anecdotally since ancient times, however only recently it has been characterized more accurately. We propose an updated overview on the main aspects of pathophysiology, genetics, diagnosis and treatment of this intriguing disease. (4) Conclusions: Overall, this review presents the all the main aspects, including real life outcome of the latest recommendation on treatment resistance of FMF, a disease, that not only helped understanding the pathophysiology of the auto inflammatory process but also the functioning of the innate immune system itself.
Keywords: Familial Mediterranean Fever; Interlukin-1 Inhibitors; autoinflammatory disease; children; colchicine; colchicine resistance.
Conflict of interest statement
M.G.: speaker fees, consultancies and unrestricted grants from Novartis and SOBI; RC speaker fees from Novartis and SOBI, SV speaker fees from SOBI.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

