Routes of Albumin Overload Toxicity in Renal Tubular Epithelial Cells
- PMID: 37298591
- PMCID: PMC10253691
- DOI: 10.3390/ijms24119640
Routes of Albumin Overload Toxicity in Renal Tubular Epithelial Cells
Abstract
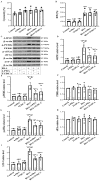
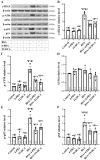
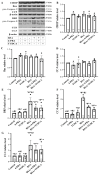
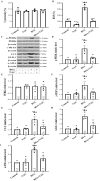
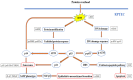
Besides being a marker of kidney disease severity, albuminuria exerts a toxic effect on renal proximal tubular epithelial cells (RPTECs). We evaluated whether an unfolded protein response (UPR) or DNA damage response (DDR) is elicited in RPTECs exposed to high albumin concentration. The deleterious outcomes of the above pathways, apoptosis, senescence, or epithelial-to-mesenchymal transition (EMT) were evaluated. Albumin caused reactive oxygen species (ROS) overproduction and protein modification, and a UPR assessed the level of crucial molecules involved in this pathway. ROS also induced a DDR evaluated by critical molecules involved in this pathway. Apoptosis ensued through the extrinsic pathway. Senescence also occurred, and the RPTECs acquired a senescence-associated secretory phenotype since they overproduced IL-1β and TGF-β1. The latter may contribute to the observed EMT. Agents against endoplasmic reticulum stress (ERS) only partially alleviated the above changes, while the inhibition of ROS upregulation prevented both UPR and DDR and all the subsequent harmful effects. Briefly, albumin overload causes cellular apoptosis, senescence, and EMT in RPTECs by triggering UPR and DDR. Promising anti-ERS factors are beneficial but cannot eliminate the albumin-induced deleterious effects because DDR also occurs. Factors that suppress ROS overproduction may be more effective since they could halt UPR and DDR.
Keywords: DNA damage; albumin; apoptosis; endoplasmic reticulum stress; epithelial-to-mesenchymal transition; renal tubular epithelial cells; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Levey A.S., de Jong P.E., Coresh J., El Nahas M., Astor B.C., Matsushita K., Gansevoort R.T., Kasiske B.L., Eckardt K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

