IGFBP7 Fuels the Glycolytic Metabolism in B-Cell Precursor Acute Lymphoblastic Leukemia by Sustaining Activation of the IGF1R-Akt-GLUT1 Axis
- PMID: 37298628
- PMCID: PMC10253689
- DOI: 10.3390/ijms24119679
IGFBP7 Fuels the Glycolytic Metabolism in B-Cell Precursor Acute Lymphoblastic Leukemia by Sustaining Activation of the IGF1R-Akt-GLUT1 Axis
Abstract
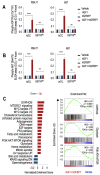
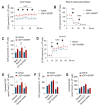
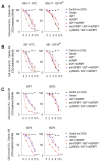
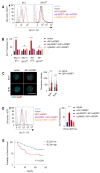
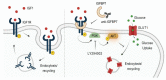
Increased glycolytic metabolism plays an important role in B-cell precursor Acute Lymphoblastic Leukemia (BCP-ALL). We previously showed that IGFBP7 exerts mitogenic and prosuvival effects in ALL by promoting IGF1 receptor (IGF1R) permanence on the cell surface, thus prolonging Akt activation upon IGFs/insulin stimulation. Here, we show that sustained activation of the IGF1R-PI3K-Akt axis concurs with GLUT1 upregulation, which enhances energy metabolism and increases glycolytic metabolism in BCP-ALL. IGFBP7 neutralization with a monoclonal antibody or the pharmacological inhibition of the PI3K-Akt pathway was shown to abrogate this effect, restoring the physiological levels of GLUT1 on the cell surface. The metabolic effect described here may offer an additional mechanistic explanation for the strong negative impact seen in ALL cells in vitro and in vivo after the knockdown or antibody neutralization of IGFBP7, while reinforcing the notion that it is a valid target for future therapeutic interventions.
Keywords: Acute Lymphoblastic Leukemia; GLUT1; IGF1R; IGFBP7; PI3K–Akt.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Maule M., Scélo G., Pastore G., Brennan P., Hemminki K., Tracey E., Sankila R., Weiderpass E., Olsen J.H., McBride M.L., et al. Risk of second malignant neoplasms after childhood leukemia and lymphoma: An international study. J. Natl. Cancer Inst. 2007;99:790–800. doi: 10.1093/jnci/djk180. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2019/04943-3/São Paulo Research Foundation
- 2022/04464-0/São Paulo Research Foundation
- 12/12802-1/São Paulo Research Foundation
- 14/20015-5/São Paulo Research Foundation
- 17/17728-8/São Paulo Research Foundation
- 305896/2013-0/National Council for Scientific and Technological Development
- 301596/2017-4/National Council for Scientific and Technological Development
- 308399/2021-8/National Council for Scientific and Technological Development
- 471003/2013-1/National Council for Scientific and Technological Development
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

