Mechanisms of Resistance to Antibody-Drug Conjugates
- PMID: 37298631
- PMCID: PMC10253543
- DOI: 10.3390/ijms24119674
Mechanisms of Resistance to Antibody-Drug Conjugates
Abstract
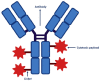
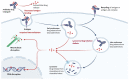
The treatment of cancer patients has dramatically changed over the past decades with the advent of monoclonal antibodies, immune-checkpoint inhibitors, bispecific antibodies, and innovative T-cell therapy. Antibody-drug conjugates (ADCs) have also revolutionized the treatment of cancer. Several ADCs have already been approved in hematology and clinical oncology, such as trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (T-DXd), and sacituzumab govitecan (SG) for the treatment of metastatic breast cancer, and enfortumab vedotin (EV) for the treatment of urothelial carcinoma. The efficacy of ADCs is limited by the emergence of resistance due to different mechanisms, such as antigen-related resistance, failure of internalization, impaired lysosomal function, and other mechanisms. In this review, we summarize the clinical data that contributed to the approval of T-DM1, T-DXd, SG, and EV. We also discuss the different mechanisms of resistance to ADCs, as well as the ways to overcome this resistance, such as bispecific ADCs and the combination of ADCs with immune-checkpoint inhibitors or tyrosine-kinase inhibitors.
Keywords: antibody-drug conjugate; enfortumab vedotin; linker; monoclonal antibodies; payload; sacituzumab govitecan; trastuzumab deruxtecan; trastuzumab emtasine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Coiffier B., Lepage E., Briere J., Herbrecht R., Tilly H., Bouabdallah R., Morel P., Van Den Neste E., Salles G., Gaulard P., et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. - DOI - PubMed
-
- Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., Fleming T., Eiermann W., Wolter J., Pegram M., et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous