Comparison of Estetrol Exposure between Women and Mice to Model Preclinical Experiments and Anticipate Human Treatment
- PMID: 37298669
- PMCID: PMC10253893
- DOI: 10.3390/ijms24119718
Comparison of Estetrol Exposure between Women and Mice to Model Preclinical Experiments and Anticipate Human Treatment
Abstract
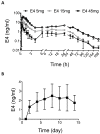
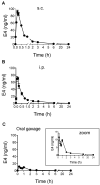
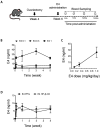
Estetrol (E4) is a natural estrogen with promising therapeutic applications in humans. The European Medicines Agency and the Food and Drug Administration have approved the use of 15 mg E4/3 mg drospirenone for contraceptive indication. Phase III clinical trials with 15-20 mg E4 for the relief of climacteric complaints are currently running. Relevant data from preclinical animal models are needed to characterize the molecular mechanisms and the pharmacological effects of E4 and possibly to reveal new therapeutic applications and to anticipate potential adverse effects. Therefore, it is important to design experimental procedures in rodents that closely mimic or anticipate human E4 exposure. In this study, we compared the effects of E4 exposure after acute or chronic administration in women and mice. Women who received chronic E4 treatment per os at a dose of 15 mg once daily reached a steady state within 6 to 8 days, with a mean plasma concentration of 3.20 ng/mL. Importantly, with subcutaneous, intraperitoneal or oral administration of E4 in mice, a stable concentration over time that would mimic human pharmacokinetics could not be achieved. The use of osmotic minipumps continuously releasing E4 for several weeks provided an exposure profile mimicking chronic oral administration in women. Measurements of the circulating concentration of E4 in mice revealed that the mouse equivalent dose necessary to mimic human treatment does not fit with the allometric prediction. In conclusion, this study highlights the importance of precise definition of the most appropriate dose and route of administration to utilize when developing predictive preclinical animal models to mimic or anticipate specific human treatment.
Keywords: estetrol; exposure; human; mice; pharmacokinetics; route of administration.
Conflict of interest statement
J.-M.F. is a member of the board of Mithra Pharmaceuticals (Belgium). M.T. and V.K. were employees of Mithra Pharmaceuticals (Belgium). A.G. was a postdoctoral fellow at the University of Liège when performing this study. Since then, she has become an employee of Mithra Pharmaceuticals (Belgium). Estetra SPRL (Belgium) sponsored the PK study in women. The other authors declare no conflict of interest with respect to this study.
Figures

References
-
- Hagen A.A., Barr M., Diczfalusy E. Metabolism of 17-Beta-Oestradiol-4-14-C in Early Infancy. Acta Endocrinol. 1965;49:207–220. - PubMed
-
- Pluchino N., Santoro A.N., Casarosa E., Giannini A., Genazzani A., Russo M., Russo N., Petignat P., Genazzani A.R. Effect of estetrol administration on brain and serum allopregnanolone in intact and ovariectomized rats. J. Steroid Biochem. Mol. Biol. 2014;143:285–290. doi: 10.1016/j.jsbmb.2014.04.011. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

