Exploring the Potential of Sulfonamide-Dihydropyridine Hybrids as Multitargeted Ligands for Alzheimer's Disease Treatment
- PMID: 37298693
- PMCID: PMC10253445
- DOI: 10.3390/ijms24119742
Exploring the Potential of Sulfonamide-Dihydropyridine Hybrids as Multitargeted Ligands for Alzheimer's Disease Treatment
Abstract
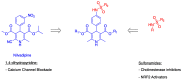
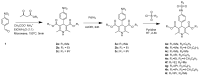
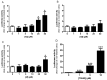
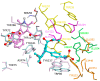
Alzheimer's disease (AD) is a multifactorial neurodegenerative disease that has a heavy social and economic impact on all societies and for which there is still no cure. Multitarget-directed ligands (MTDLs) seem to be a promising therapeutic strategy for finding an effective treatment for this disease. For this purpose, new MTDLs were designed and synthesized in three steps by simple and cost-efficient procedures targeting calcium channel blockade, cholinesterase inhibition, and antioxidant activity. The biological and physicochemical results collected in this study allowed us the identification two sulfonamide-dihydropyridine hybrids showing simultaneous cholinesterase inhibition, calcium channel blockade, antioxidant capacity and Nrf2-ARE activating effect, that deserve to be further investigated for AD therapy.
Keywords: Hantzsch reaction; Nrf2; calcium channel antagonism; cholinesterase inhibition; multitarget directed ligands.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
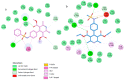
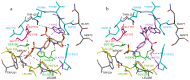
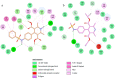
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

