Stretch-Induced Down-Regulation of HCN2 Suppresses Contractile Activity
- PMID: 37298834
- PMCID: PMC10254498
- DOI: 10.3390/molecules28114359
Stretch-Induced Down-Regulation of HCN2 Suppresses Contractile Activity
Abstract
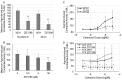
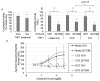
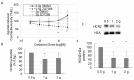
Although hyperpolarization-activated and cyclic nucleotide-gated 2 channels (HCN2) are expressed in multiple cell types in the gut, the role of HCN2 in intestinal motility is poorly understood. HCN2 is down-regulated in intestinal smooth muscle in a rodent model of ileus. Thus, the purpose of this study was to determine the effects of HCN inhibition on intestinal motility. HCN inhibition with ZD7288 or zatebradine significantly suppressed both spontaneous and agonist-induced contractile activity in the small intestine in a dose-dependent and tetrodotoxin-independent manner. HCN inhibition significantly suppressed intestinal tone but not contractile amplitude. The calcium sensitivity of contractile activity was significantly suppressed by HCN inhibition. Inflammatory mediators did not affect the suppression of intestinal contractile activity by HCN inhibition but increased stretch of the intestinal tissue partially attenuated the effects of HCN inhibition on agonist-induced intestinal contractile activity. HCN2 protein and mRNA levels in intestinal smooth muscle tissue were significantly down-regulated by increased mechanical stretch compared to unstretched tissue. Increased cyclical stretch down-regulated HCN2 protein and mRNA levels in primary human intestinal smooth muscle cells and macrophages. Overall, our results suggest that decreased HCN2 expression induced by mechanical signals, such as intestinal wall distension or edema development, may contribute to the development of ileus.
Keywords: hyperpolarization-activated and cyclic nucleotide-gated 2 channel; ileus; intestinal motility; mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Role of hyperpolarization-activated cyclic nucleotide-gated channel HCN2 in embryonic neural stem cell proliferation and differentiation.Neurochem Int. 2022 Oct;159:105387. doi: 10.1016/j.neuint.2022.105387. Epub 2022 Jul 11. Neurochem Int. 2022. PMID: 35835292
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
In vitro characterization of HCN channel kinetics and frequency dependence in myocytes predicts biological pacemaker functionality.J Physiol. 2009 Apr 1;587(Pt 7):1513-25. doi: 10.1113/jphysiol.2008.163444. Epub 2009 Jan 26. J Physiol. 2009. PMID: 19171659 Free PMC article.
-
Hyperpolarization-activated and cyclic nucleotide-gated channel proteins as emerging new targets in neuropathic pain.Rev Neurosci. 2019 Jul 26;30(6):639-649. doi: 10.1515/revneuro-2018-0094. Rev Neurosci. 2019. PMID: 30768426 Review.
-
HCN channelopathies: pathophysiology in genetic epilepsy and therapeutic implications.Br J Pharmacol. 2012 Jan;165(1):49-56. doi: 10.1111/j.1476-5381.2011.01507.x. Br J Pharmacol. 2012. PMID: 21615728 Free PMC article. Review.
References
-
- Grocott M.P., Browne J.P., Van der Meulen J., Matejowsky C., Mutch M., Hamilton M.A., Levett D.Z., Emberton M., Haddad F.S., Mythen M.G. The Postoperative Morbidity Survey was validated and used to describe morbidity after major surgery. J. Clin. Epidemiol. 2007;60:919–928. doi: 10.1016/j.jclinepi.2006.12.003. - DOI - PubMed
-
- Solanki S., Chakinala R.C., Haq K.F., Singh J., Khan M.A., Solanki D., Vyas M.J., Kichloo A., Mansuri U., Shah H., et al. Paralytic ileus in the United States: A cross-sectional study from the national inpatient sample. SAGE Open Med. 2020;8:2050312120962636. doi: 10.1177/2050312120962636. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

