Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa
- PMID: 37298857
- PMCID: PMC10254538
- DOI: 10.3390/molecules28114379
Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa
Abstract
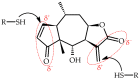
Arnica montana is a valuable plant with high demand on the pharmaceutical and cosmetic market due to the presence of helenalin (H) and 11α, 13-dihydrohelenalin (DH) sesquiterpene lactones (SLs), with many applications and anti-inflammatory, anti-tumor, analgesic and other properties. Despite the great importance of these compounds for the protection of the plant and their medicinal value, the content of these lactones and the profile of the compounds present within individual elements of florets and flower heads have not been studied so far, and attempts to localize these compounds in flower tissues have also not been conducted. The three studied Arnica taxa synthesize SLs only in the aerial parts of plants, and the highest content of these substances was found in A. montana cv. Arbo; it was lower in wild species, and a very small amount of H was produced by A. chamissonis. Analysis of dissected fragments of whole inflorescences revealed a specific distribution pattern of these compounds. The lactones content in single florets increased from the top of the corolla to the ovary, with the pappus calyx being a significant source of their production. Histochemical tests for terpenes and methylene ketones indicated the colocalization of lactones with inulin vacuoles.
Keywords: A. chamissonis; Arnica montana; biosynthesis localization; helenalin and dihydrohelenalin esters; histochemical study; sesquiterpene lactones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Arnica montana L.: Doesn't Origin Matter?Plants (Basel). 2023 Oct 11;12(20):3532. doi: 10.3390/plants12203532. Plants (Basel). 2023. PMID: 37895999 Free PMC article. Review.
-
Quantitative analysis of sesquiterpene lactones in extract of Arnica montana L. by 1H NMR spectroscopy.J Pharm Biomed Anal. 2011 Jan 5;54(1):94-9. doi: 10.1016/j.jpba.2010.08.018. Epub 2010 Aug 21. J Pharm Biomed Anal. 2011. PMID: 20837387
-
Sesquiterpene lactones in Arnica montana: helenalin and dihydrohelenalin chemotypes in Spain.Planta Med. 2009 May;75(6):660-6. doi: 10.1055/s-0029-1185362. Epub 2009 Feb 23. Planta Med. 2009. PMID: 19235681
-
Sesquiterpene lactones in Arnica montana: a rapid analytical method and the effects of flower maturity and simulated mechanical harvesting on quality and yield.Planta Med. 2004 Feb;70(2):166-70. doi: 10.1055/s-2004-815495. Planta Med. 2004. PMID: 14994196
-
Final report on the safety assessment of Arnica montana extract and Arnica montana.Int J Toxicol. 2001;20 Suppl 2:1-11. doi: 10.1080/10915810160233712. Int J Toxicol. 2001. PMID: 11558636 Review.
Cited by
-
Arnica montana L.: Doesn't Origin Matter?Plants (Basel). 2023 Oct 11;12(20):3532. doi: 10.3390/plants12203532. Plants (Basel). 2023. PMID: 37895999 Free PMC article. Review.
-
Impact of elicitors and light on biosynthesis of sesquiterpene lactones in tissue culture of Arnica montana and its variety Arbo.Front Plant Sci. 2025 Jun 19;16:1611849. doi: 10.3389/fpls.2025.1611849. eCollection 2025. Front Plant Sci. 2025. PMID: 40612603 Free PMC article.
-
Interactions of Highly Diluted Arnica montana Extract with Water Across Glass Interfaces.Int J Mol Sci. 2025 Jan 27;26(3):1115. doi: 10.3390/ijms26031115. Int J Mol Sci. 2025. PMID: 39940883 Free PMC article.
-
Natural sesquiterpene lactones in prostate cancer therapy: mechanisms and sources.Med Oncol. 2025 May 15;42(6):212. doi: 10.1007/s12032-025-02740-2. Med Oncol. 2025. PMID: 40372575 Review.
References
-
- Rodríguez-Chávez J.L., Egas V., Linares E., Bye R., Hernández T., Espinosa-García F.J., Delgado G. Mexican Arnica (Heterotheca Inuloides Cass. Asteraceae: Astereae): Ethnomedical Uses, Chemical Constituents and Biological Properties. J. Ethnopharmacol. 2017;195:39–63. doi: 10.1016/j.jep.2016.11.021. - DOI - PubMed
-
- Duthen S., Gadéa A., Trempat P., Boujedaini N., Fabre N. Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules. 2022;27:2737. doi: 10.3390/molecules27092737. - DOI - PMC - PubMed
-
- Jones C.G., Keeling C.I., Ghisalberti E.L., Barbour E.L., Plummer J.A., Bohlmann J. Isolation of CDNAs and Functional Characterisation of Two Multi-Product Terpene Synthase Enzymes from Sandalwood, Santalum album L. Arch. Biochem. Biophys. 2008;477:121–130. doi: 10.1016/j.abb.2008.05.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

