New Functions of Intracellular LOXL2: Modulation of RNA-Binding Proteins
- PMID: 37298909
- PMCID: PMC10254187
- DOI: 10.3390/molecules28114433
New Functions of Intracellular LOXL2: Modulation of RNA-Binding Proteins
Abstract
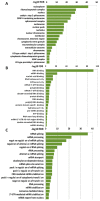
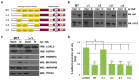
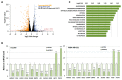
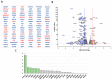
Lysyl oxidase-like 2 (LOXL2) was initially described as an extracellular enzyme involved in extracellular matrix remodeling. Nevertheless, numerous recent reports have implicated intracellular LOXL2 in a wide variety of processes that impact on gene transcription, development, differentiation, proliferation, migration, cell adhesion, and angiogenesis, suggesting multiple different functions for this protein. In addition, increasing knowledge about LOXL2 points to a role in several types of human cancer. Moreover, LOXL2 is able to induce the epithelial-to-mesenchymal transition (EMT) process-the first step in the metastatic cascade. To uncover the underlying mechanisms of the great variety of functions of intracellular LOXL2, we carried out an analysis of LOXL2's nuclear interactome. This study reveals the interaction of LOXL2 with numerous RNA-binding proteins (RBPs) involved in several aspects of RNA metabolism. Gene expression profile analysis of cells silenced for LOXL2, combined with in silico identification of RBPs' targets, points to six RBPs as candidates to be substrates of LOXL2's action, and that deserve a more mechanistic analysis in the future. The results presented here allow us to hypothesize novel LOXL2 functions that might help to comprehend its multifaceted role in the tumorigenic process.
Keywords: EMT; LOXL2; RNA-binding proteins; intracellular LOXL2; nuclear interactome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

