Design, Synthesis and Anticancer Evaluation of Nitroimidazole Radiosensitisers
- PMID: 37298933
- PMCID: PMC10254852
- DOI: 10.3390/molecules28114457
Design, Synthesis and Anticancer Evaluation of Nitroimidazole Radiosensitisers
Abstract
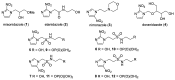
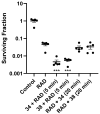
The role of hypoxic tumour cells in resistance to radiotherapy, and in suppression of immune response, continues to endorse tumour hypoxia as a bona fide, yet largely untapped, drug target. Radiotherapy innovations such as stereotactic body radiotherapy herald new opportunities for classical oxygen-mimetic radiosensitisers. Only nimorazole is used clinically as a radiosensitiser, and there is a dearth of new radiosensitisers in development. In this report, we augment previous work to present new nitroimidazole alkylsulfonamides and we document their cytotoxicity and ability to radiosensitise anoxic tumour cells in vitro. We compare radiosensitisation with etanidazole and earlier nitroimidazole sulfonamide analogues and we identify 2-nitroimidazole and 5-nitroimidazole analogues with marked tumour radiosensitisation in ex vivo assays of surviving clonogens and with in vivo tumour growth inhibition.
Keywords: DNA damage; chemoradiotherapy; electron affinity; hypoxia; nitroimidazole; prodrugs; radiosensitisers; radiotherapy; sulfonamide; tumour microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

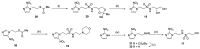


References
-
- Pignon J.P., Bourhis J., Domenge C., Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. - PubMed
-
- Bentzen S.M., Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J. Clin. Oncol. 2007;25:4096–4103. - PubMed
-
- Harrington K.J., Billingham L.J., Brunner T.B., Chan C.S., Hoskin P., Mackay R.I., Maughan T.S., J Macdougall J., McKenna W.G., Nutting C.M., et al. Guidelines for preclinical and early phase clinical assessment of novel radiosensitisers. Br. J. Cancer. 2011;105:628–639. doi: 10.1038/bjc.2011.240. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

