Synthetic Study toward Triterpenes from the Schisandraceae Family of Natural Products
- PMID: 37298943
- PMCID: PMC10254518
- DOI: 10.3390/molecules28114468
Synthetic Study toward Triterpenes from the Schisandraceae Family of Natural Products
Abstract
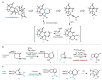
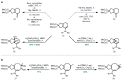
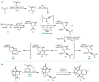
Triterpenoid natural products from the Schisandraceae family have long presented a significant synthetic challenge. Lancifodilactone I, a member of the family not previously synthesized, was identified as a key natural product target, from which many other members could be synthesized. We envisaged that the core ring system of lancifodilactone I could be accessed by a strategy involving palladium-catalysed cascade cyclisation of a bromoenynamide, via carbopalladation, Suzuki coupling and 8π-electrocyclisation, to synthesize the core 7,8-fused ring system. Exploration of this strategy on model systems resulted in efficient syntheses of 5,6- and 5,8-fused systems in high yields, which represent the first such cyclisation where the ynamide nitrogen atom is 'external' to the forming ring system. The enamide functionality resident in the cascade cyclisation product was found to be less nucleophilic than the accompanying tri-/tetrasubstituted alkene(s), enabling regioselective oxidations. Application of this strategy to 7,6-, and 7,8-fused systems, and ultimately the 'real' substrate, was ultimately thwarted by the difficulty of 7-membered ring closure, leading to side product formation. Nevertheless, a tandem bromoenynamide carbopalladation, Suzuki coupling and 6/8π-electrocyclisation was shown to be a highly efficient tactic for the formation of bicyclic enamides, which may find applications in other synthetic contexts.
Keywords: Suzuki coupling; cascade reaction; electrocyclisation; medium ring synthesis; natural products; total synthesis; ynamides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

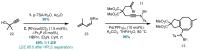
Similar articles
-
The Journey of Schinortriterpenoid Total Syntheses.Acc Chem Res. 2019 Feb 19;52(2):480-491. doi: 10.1021/acs.accounts.8b00569. Epub 2019 Jan 25. Acc Chem Res. 2019. PMID: 30681828
-
A palladium-mediated cascade cyclisation approach to the CDE cores of rubriflordilactone A and lancifodilactone G.Chem Commun (Camb). 2008 Nov 30;(44):5818-20. doi: 10.1039/b814360a. Epub 2008 Oct 2. Chem Commun (Camb). 2008. PMID: 19009092
-
Ynamide carbopalladation: a flexible route to mono-, bi- and tricyclic azacycles.Chemistry. 2015 Sep 1;21(36):12627-39. doi: 10.1002/chem.201501710. Epub 2015 Jul 16. Chemistry. 2015. PMID: 26189754 Free PMC article.
-
[Studies on the Syntheses of Bioactive Natural Products Having a Fused Ring System Based on Novel Skeletal Construction Methods].Yakugaku Zasshi. 2021;141(9):1087-1094. doi: 10.1248/yakushi.21-00126. Yakugaku Zasshi. 2021. PMID: 34471010 Review. Japanese.
-
Recent advances in 8π electrocyclization reactions.Chem Commun (Camb). 2023 Jan 17;59(6):670-687. doi: 10.1039/d2cc04805a. Chem Commun (Camb). 2023. PMID: 36597987 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

