Europium(III) Meets Etidronic Acid (HEDP): A Coordination Study Combining Spectroscopic, Spectrometric, and Quantum Chemical Methods
- PMID: 37298946
- PMCID: PMC10254137
- DOI: 10.3390/molecules28114469
Europium(III) Meets Etidronic Acid (HEDP): A Coordination Study Combining Spectroscopic, Spectrometric, and Quantum Chemical Methods
Abstract
Etidronic acid (1-Hydroxyethylidene-1,1-diphosphonic acid, HEDP, H4L) is a proposed decorporation agent for U(VI). This paper studied its complex formation with Eu(III), an inactive analog of trivalent actinides, over a wide pH range, at varying metal-to-ligand ratios (M:L) and total concentrations. Combining spectroscopic, spectrometric, and quantum chemical methods, five distinct Eu(III)-HEDP complexes were found, four of which were characterized. The readily soluble EuH2L+ and Eu(H2L)2- species with log β values of 23.7 ± 0.1 and 45.1 ± 0.9 are formed at acidic pH. At near-neutral pH, EuHL0s forms with a log β of ~23.6 and, additionally, a most probably polynuclear complex. The readily dissolved EuL- species with a log β of ~11.2 is formed at alkaline pH. A six-membered chelate ring is the key motif in all solution structures. The equilibrium between the Eu(III)-HEDP species is influenced by several parameters, i.e., pH, M:L, total Eu(III) and HEDP concentrations, and time. Overall, the present work sheds light on the very complex speciation in the HEDP-Eu(III) system and indicates that, for risk assessment of potential decorporation scenarios, side reactions of HEDP with trivalent actinides and lanthanides should also be taken into account.
Keywords: ATR-FT-IR; DFT; ESI-MS; NMR; TRLFS; complexation; lanthanides; speciation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
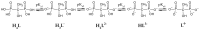



Similar articles
-
Curium(III) citrate speciation in biological systems: a europium(III) assisted spectroscopic and quantum chemical study.Dalton Trans. 2012 Dec 7;41(45):13969-83. doi: 10.1039/c2dt31480k. Epub 2012 Oct 1. Dalton Trans. 2012. PMID: 23027503
-
Caffeic Acid/Eu(III) Complexes: Solution Equilibrium Studies, Structure Characterization and Biological Activity.Int J Mol Sci. 2022 Jan 14;23(2):888. doi: 10.3390/ijms23020888. Int J Mol Sci. 2022. PMID: 35055074 Free PMC article.
-
Speciation and spatial distribution of Eu(III) in fungal mycelium.Sci Total Environ. 2022 Dec 10;851(Pt 2):158160. doi: 10.1016/j.scitotenv.2022.158160. Epub 2022 Aug 19. Sci Total Environ. 2022. PMID: 35988601
-
Speciation of the trivalent f-elements Eu(III) and Cm(III) in digestive media.J Inorg Biochem. 2017 Oct;175:248-258. doi: 10.1016/j.jinorgbio.2017.07.020. Epub 2017 Aug 4. J Inorg Biochem. 2017. PMID: 28802224
-
Sorption speciation of lanthanides/actinides on minerals by TRLFS, EXAFS and DFT studies: a review.Molecules. 2010 Nov 17;15(11):8431-68. doi: 10.3390/molecules15118431. Molecules. 2010. PMID: 21085087 Free PMC article. Review.
Cited by
-
Performance of ionic liquid functionalized metal organic frameworks in the adsorption process of phenol derivatives.RSC Adv. 2024 Feb 5;14(7):4759-4777. doi: 10.1039/d3ra08024b. eCollection 2024 Jan 31. RSC Adv. 2024. PMID: 38318619 Free PMC article.
-
Equilibrium Thermodynamics of Macropa Complexes with Selected Metal Isotopes of Radiopharmaceutical Interest.Inorg Chem. 2023 Dec 18;62(50):20699-20709. doi: 10.1021/acs.inorgchem.3c01983. Epub 2023 Sep 13. Inorg Chem. 2023. PMID: 37702665 Free PMC article.
-
Preparation and Evaluation of a Combination of Chelating Agents for the Removal of Inhaled Uranium.Molecules. 2024 Dec 5;29(23):5759. doi: 10.3390/molecules29235759. Molecules. 2024. PMID: 39683918 Free PMC article.
References
-
- Farooqi I.H., Hussain A., Quraishi M.A., Saini P.A. Study of low cost eco-friendly compounds as corrosion inhibitors for cooling systems. Anti-Corros. Methods Mater. 1999;46:328–331. doi: 10.1108/00035599910295508. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

