Benzoylaconitine Alleviates Progression of Psoriasis via Suppressing STAT3 Phosphorylation in Keratinocytes
- PMID: 37298949
- PMCID: PMC10254579
- DOI: 10.3390/molecules28114473
Benzoylaconitine Alleviates Progression of Psoriasis via Suppressing STAT3 Phosphorylation in Keratinocytes
Abstract
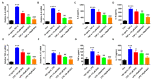
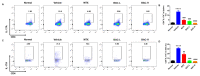
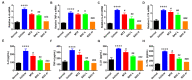
Psoriasis is a chronic and multifactorial skin disease which is caused by inflammatory infiltrates, keratinocyte hyperproliferation, and accumulation of immune cells. As part of the Aconitum species, Benzoylaconitine (BAC) shows potential antiviral, anti-tumor, and anti-inflammatory effects. In this study, we investigated the effects and mechanisms of BAC on tumor necrosis factor-alpha (TNF-α)/LPS-induced HaCaT keratinocytes in a imiquimod(IMQ)-induced mice model. The results showed that BAC could relieve the symptoms of psoriasis by inhibiting cell proliferation, the release of inflammatory factors, and the accumulation of Th17 cells, while no obvious effect on cell viability and safety was observed both in vitro and in vivo. Additionally, BAC can markedly inhibit the protein and mRNA levels of inflammatory cytokines in TNF-α/LPS-induced HaCaT keratinocytes by inhibiting the phosphorylation of STAT3. In brief, our data indicated that BAC could alleviate the progression of psoriasis and may be a potential therapeutic agent for treating psoriasis in clinical practice.
Keywords: Benzoylaconitine; HaCaT keratinocytes; STAT3 signaling; inflammatory cytokine; psoriasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

