The Benzothiazine Core as a Novel Motif for DNA-Binding Small Molecules
- PMID: 37298974
- PMCID: PMC10254314
- DOI: 10.3390/molecules28114499
The Benzothiazine Core as a Novel Motif for DNA-Binding Small Molecules
Abstract
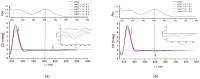
A new series of 4H-1,3-benzothiazine dyes were prepared and fully characterized in an aqueous medium. Benzothiazine salts were synthesized either through the classical synthetic pathway using Buchwald-Hartwig amination or through economical and environmentally friendly electrochemical synthesis. The latest synthetic approach employs successful electrochemical intramolecular dehydrogenative cyclization of N-benzylbenzenecarbothioamides to form 4H-1,3-benzothiazines. 4H-1,3-Benzothiazines were evaluated as novel DNA/RNA probes. Through the use of several methods such as UV/vis spectrophotometric titrations, circular dichroism and thermal melting experiments, the binding of four benzothiazine-based molecules to polynucleotides was examined. Compounds 1 and 2 acted as DNA/RNA groove binders, thus suggesting the potential of these compounds as novel DNA/RNA probes. This is a proof-of-concept study and will be expanded to include SAR/QSAR studies.
Keywords: 4H-1,3-benzothiazines; DNA/RNA binding; UV/vis spectroscopy; circular dichroism; electrochemical dehydrogenative cyclization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


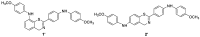



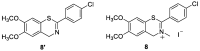



References
-
- Szczęśniak-Sięga B.M., Wiatrak B., Czyżnikowska Ż., Janczak J., Wiglusz R.J., Maniewska J. Synthesis and biological evaluation as well as in silico studies of arylpiperazine-1, 2-benzothiazine derivatives as novel anti-inflammatory agents. Bioorg. Chem. 2021;106:104476. doi: 10.1016/j.bioorg.2020.104476. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

