Recent Progress in the Integration of CO2 Capture and Utilization
- PMID: 37298975
- PMCID: PMC10254406
- DOI: 10.3390/molecules28114500
Recent Progress in the Integration of CO2 Capture and Utilization
Abstract
CO2 emission is deemed to be mainly responsible for global warming. To reduce CO2 emissions into the atmosphere and to use it as a carbon source, CO2 capture and its conversion into valuable chemicals is greatly desirable. To reduce the transportation cost, the integration of the capture and utilization processes is a feasible option. Here, the recent progress in the integration of CO2 capture and conversion is reviewed. The absorption, adsorption, and electrochemical separation capture processes integrated with several utilization processes, such as CO2 hydrogenation, reverse water-gas shift reaction, or dry methane reforming, is discussed in detail. The integration of capture and conversion over dual functional materials is also discussed. This review is aimed to encourage more efforts devoted to the integration of CO2 capture and utilization, and thus contribute to carbon neutrality around the world.
Keywords: CO2 capture; CO2 conversion; carbon neutrality; integration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
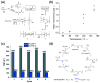

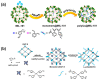


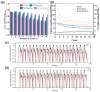
References
-
- Zhang P., Tong J., Huang K., Zhu X., Yang W. The current status of high temperature electrochemistry-based CO2 transport membranes and reactors for direct CO2 capture and conversion. Prog. Energy Combust. Sci. 2021;82:100888. doi: 10.1016/j.pecs.2020.100888. - DOI
-
- Yamada H. Amine-based capture of CO2 for utilization and storage. Polym. J. 2021;53:93–102. doi: 10.1038/s41428-020-00400-y. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

