Methylene Blue Dye Adsorption on Iron Oxide-Hydrochar Composite Synthesized via a Facile Microwave-Assisted Hydrothermal Carbonization of Pomegranate Peels' Waste
- PMID: 37299002
- PMCID: PMC10254837
- DOI: 10.3390/molecules28114526
Methylene Blue Dye Adsorption on Iron Oxide-Hydrochar Composite Synthesized via a Facile Microwave-Assisted Hydrothermal Carbonization of Pomegranate Peels' Waste
Abstract
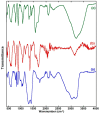
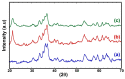
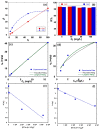
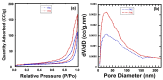
The toxicity of dyes has a long-lasting negative impact on aquatic life. Adsorption is an inexpensive, simple, and straightforward technique for eliminating pollutants. One of the challenges facing adsorption is that it is hard to collect the adsorbents after the adsorption. Adding a magnetic property to the adsorbents makes it easier to collect the adsorbents. The current work reports the synthesis of an iron oxide-hydrochar composite (FHC) and an iron oxide-activated hydrochar composite (FAC) through the microwave-assisted hydrothermal carbonization (MHC) technique, which is known as a timesaving and energy-efficient method. The synthesized composites were characterized using various techniques, such as FT-IR, XRD, SEM, TEM, and N2 isotherm. The prepared composites were applied in the adsorption of cationic methylene blue dye (MB). The composites were formed of crystalline iron oxide and amorphous hydrochar, with a porous structure for the hydrochar and a rod-like structure for the iron oxide. The pH of the point of zero charge (pHpzc) of the iron oxide-hydrochar composite and the iron oxide-activated hydrochar composite were 5.3 and 5.6, respectively. Approximately 556 mg and 50 mg of MB dye was adsorbed on the surface of 1 g of the FHC and FAC, respectively, according to the maximum adsorption capacity calculated using the Langmuir model.
Keywords: adsorption; iron oxide-hydrochar composites; methylene blue dye; microwave-assisted hydrothermal carbonization method; pomegranate peels.
Conflict of interest statement
The author declares no conflict of interest.
Figures





References
-
- HasdemİR Z.M., ŞİMŞEk S. Removal of Cationic Dye in Aquatic Medium by Using a New Composite Material. Cumhur. Sci. J. 2018;39:181–191. doi: 10.17776/csj.356915. - DOI
-
- Yu F., Tian F., Zou H., Ye Z., Peng C., Huang J., Zheng Y., Zhang Y., Yang Y., Wei X., et al. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021;415:125511. doi: 10.1016/j.jhazmat.2021.125511. - DOI - PubMed
-
- Pauletto P.S., Moreno-Perez J., Hernandez-Hernandez L.E., Bonilla-Petriciolet A., Dotto G.L., Salau N.P.G. Novel biochar and hydrochar for the adsorption of 2-nitrophenol from aqueous solutions: An approach using the PVSDM model. Chemosphere. 2021;269:128748. doi: 10.1016/j.chemosphere.2020.128748. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

