Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives
- PMID: 37299013
- PMCID: PMC10254235
- DOI: 10.3390/molecules28114537
Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives
Abstract
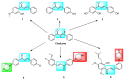
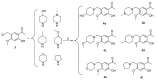
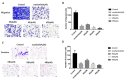
This study involved the design and synthesis of 21 new nitrogen-containing heterocyclic chalcone derivatives utilizing the active substructure splicing principle, with glycyrrhiza chalcone serving as the lead compound. The targets of these derivatives were VEGFR-2 and P-gp, and their efficacy against cervical cancer was evaluated. Following preliminary conformational analysis, compound 6f ((E)-1-(2-hydroxy-5-((4-hydroxypiperidin-1-yl)methyl)-4-methoxyphenyl)-3-(4-((4-methylpiperidin-1-yl)methyl)phenyl)prop-2-en-1-one) exhibited significant antiproliferative activity against human cervical cancer cells (HeLa and SiHa) with IC50 values of 6.52 ± 0.42 and 7.88 ± 0.52 μM, respectively, when compared to other compounds and positive control drugs. Additionally, this compound demonstrated lower toxicity towards human normal cervical epithelial cells (H8). Subsequent investigations have demonstrated that 6f exerts an inhibitory impact on VEGFR-2, as evidenced by its ability to impede the phosphorylation of p-VEGFR-2, p-PI3K, and p-Akt proteins in HeLa cells. This, in turn, results in the suppression of cell proliferation and the induction of both early and late apoptosis in a concentration-dependent manner. Furthermore, 6f significantly curtails the invasion and migration of HeLa cells. In addition, 6f had an IC50 of 7.74 ± 0.36 μM against human cervical cancer cisplatin-resistant HeLa/DDP cells and a resistance index (RI) of 1.19, compared to 7.36 for cisplatin HeLa cells. The combination of 6f and cisplatin resulted in a significant reduction in cisplatin resistance in HeLa/DDP cells. Molecular docking analyses revealed that 6f exhibited binding free energies of -9.074 and -9.823 kcal·mol-1 to VEGFR-2 and P-gp targets, respectively, and formed hydrogen bonding forces. These findings suggest that 6f has potential as an anti-cervical cancer agent and may reverse cisplatin-resistant activity in cervical cancer. The introduction of the 4-hydroxy piperidine and 4-methyl piperidine rings may contribute to its efficacy, and its mechanism of action may involve dual inhibition of VEGFR-2 and P-gp targets.
Keywords: anti-cervical cancer activity; azacyclic; cisplatin resistance; glycyrrhiza chalcone; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





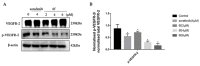



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

