1,3-Butadiynamides the Ethynylogous Ynamides: Synthesis, Properties and Applications in Heterocyclic Chemistry
- PMID: 37299038
- PMCID: PMC10254211
- DOI: 10.3390/molecules28114564
1,3-Butadiynamides the Ethynylogous Ynamides: Synthesis, Properties and Applications in Heterocyclic Chemistry
Abstract
1,3-butadiynamides-the ethynylogous variants of ynamides-receive considerable attention as precursors of complex molecular scaffolds for organic and heterocyclic chemistry. The synthetic potential of these C4-building blocks reveals itself in sophisticated transition-metal catalyzed annulation reactions and in metal-free or silver-mediated HDDA (Hexa-dehydro-Diels-Alder) cycloadditions. 1,3-Butadiynamides also gain significance as optoelectronic materials and in less explored views on their unique helical twisted frontier molecular orbitals (Hel-FMOs). The present account summarizes different methodologies for the synthesis of 1,3-butadiynamides followed by the description of their molecular structure and electronic properties. Finally, the surprisingly rich chemistry of 1,3-butadiynamides as versatile C4-building blocks in heterocyclic chemistry is reviewed by compiling their exciting reactivity, specificity and opportunities for organic synthesis. Besides chemical transformations and use in synthesis, a focus is set on the mechanistic understanding of the chemistry of 1,3-butadiynamides-suggesting that 1,3-butadiynamides are not just simple alkynes. These ethynylogous variants of ynamides have their own molecular character and chemical reactivity and reflect a new class of remarkably useful compounds.
Keywords: 1,3-diynamides; Hexa-dehydro-Diels–Alder (HDDA) reaction; alkynes; annulation reactions; carbazoles; cycloaddition cascade reactions; helical twisted frontier molecular orbitals (Hel-FMO); heterocycles; homogeneous catalysis; indoles; quinolines; ynamides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















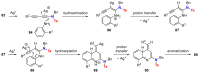
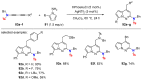
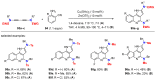

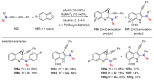
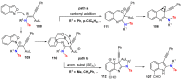

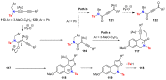
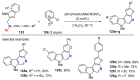
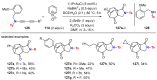













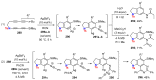
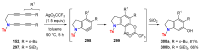

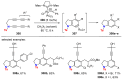

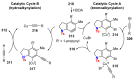
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

