Comparative MS- and NMR-Based Metabolome Mapping of Egyptian Red and White Squill Bulbs F. Liliaceae and in Relation to Their Cytotoxic Effect
- PMID: 37299060
- PMCID: PMC10255078
- DOI: 10.3390/plants12112078
Comparative MS- and NMR-Based Metabolome Mapping of Egyptian Red and White Squill Bulbs F. Liliaceae and in Relation to Their Cytotoxic Effect
Abstract
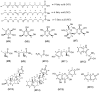
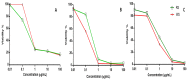
Urginea maritima L. (squill) species is widely spread at the Mediterranean region as two main varieties, i.e., white squill (WS) and red squill (RS), that are recognized for several health potentials. The major secondary metabolite classes of the squill are cardiac glycosides, mainly, bufadienolides, flavonoids, and anthocyanins. Herein, a multiplex MS and NMR metabolomics approach targeting secondary and aroma compounds in WS and RS was employed for varieties classification. Solid-phase micro extraction-gas chromatography/mass spectroscopy (SPME-GC/MS), ultra-high-performance liquid chromatography/mass spectrometry (UPLC/MS), as well as nuclear magnetic resonance (NMR) provided fingerprinting and structural confirmation of the major metabolites for both types of the squill. For comparison of the different platforms' classification potential, multivariate data analysis was employed. While Bufadienolides, viz. "hydroxy-scilliglaucosidin-O-rhamnoside, desacetylscillirosidin-O-rhamnoside and bufotalidin-O-hexoside" as well as oxylipids, were enriched in WS, flavonoids, i.e., dihydro-kaempferol-O-hexoside and its aglycon, taxifolin derivative, were predominant in RS. A cytotoxicity screening against three cancer cell lines, including breast adenocarcinoma (MCF-7), lung (A-549), and ovarian (SKOV-3) cell lines was conducted. Results revealed that WS was more effective on A-549 and SKOV-3 cell lines (WS IC50 0.11 and 0.4 µg/mL, respectively) owing to its abundance of bufadienolides, while RS recorded IC50 (MCF7 cell line) 0.17 µg/mL since is is rich inflavonoids.
Keywords: NMR; SPME-GC/MS; UPLC/MS; Urginea martima; bufadienolides; metabolomics; squill.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- El-Seedi H.R., Burman R., Mansour A., Turki Z., Boulos L., Gullbo J., Goransson U. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol. 2013;145:746–757. doi: 10.1016/j.jep.2012.12.007. - DOI - PubMed
-
- BayazıT V., Konar V. Analgesic Effects of Scilliroside, Proscillaridin-A and Taxifolin from Squill Bulb (Urginea maritima) on Pains. Dig. J. Nanomater. Biostruct. (DJNB) 2010;5:457–465.
-
- Dizaye K., Hamed B. Cardiovascular studies of white squill (Urginea maritima) extract. Zanco J. Med. Sci. 2010;14:20–27.
-
- Leblanc F.J., Lee C.O. A study of the toxic principles of red squill. J. Am. Pharm. Assoc. 1939;28:151–154.
LinkOut - more resources
Full Text Sources
Miscellaneous

