Silver Nanoparticles of Artemisia sieberi Extracts: Chemical Composition and Antimicrobial Activities
- PMID: 37299074
- PMCID: PMC10255823
- DOI: 10.3390/plants12112093
Silver Nanoparticles of Artemisia sieberi Extracts: Chemical Composition and Antimicrobial Activities
Abstract
Background: Artemisia sieberi (mugwort) is a member of the daisy family Asteraceae and is widely propagated in Saudi Arabia. A. sieberi has historical medical importance in traditional societies. The current study aimed to assess the antibacterial and antifungal characteristics of the aqueous and ethanolic extracts of A. sieberi. In addition, the study investigated the effect of silver nanoparticles (AgNPs) synthesized from the A. sieberi extract.
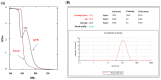
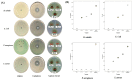
Methods: The ethanolic and aqueous extracts and AgNPs were prepared from the shoots of A. sieberi. The characteristics of AgNPs were assessed by UV-visible spectroscopy, transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR), and dynamic light scattering (DLS). The antibacterial experiments were performed against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa. The fungal species used were Candida parapsilosis, Candida krusei, Candida famata, Candida rhodotorula, and Candida albicans. The antibacterial and antifungal characteristics were evaluated by measuring the diameter of growing organisms in Petri dishes treated with different concentrations of either extracts or AgNPs compared to the untreated controls. Furthermore, TEM imaging was used to investigate any ultrastructure changes in the microbes treated with crude extracts and AgNO3.
Results: The ethanolic and aqueous extracts significantly decreased the growth of E. coli, S. aureus, and B. subtilis (p < 0.001), while P. aeruginosa was not affected. Unlike crude extracts, AgNPs had more substantial antibacterial effects against all species. In addition, the mycelial growth of C. famata was reduced by the treatment of both extracts. C. krusei mycelial growth was decreased by the aqueous extract, while the growth of C. parapsilosis was affected by the ethanolic extract and AgNPs (p < 0.001). None of the treatments affected the growth of C. albicans or C. rhodotorula. TEM analysis showed cellular ultrastructure changes in the treated S. aureus and C. famata compared to the control.
Conclusion: The biosynthesized AgNPs and extracts of A. sieberi have a potential antimicrobial characteristic against pathogenic bacterial and fungal strains and nullified resistance behavior.
Keywords: Artemisia sieberi; TEM analysis; antibacterial; antifungal; pathogenic microbes; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Batiha G.E.-S., Beshbishy A.M., Tayebwa D.S., Adeyemi O.S., Yokoyama N., Igarashi I. Anti-piroplasmic potential of the methanolic Peganum harmala seeds and ethanolic Artemisia absinthium leaf extracts. J. Protozool. Res. 2019;29:8–25.
-
- Batiha G.E.-S., Olatunde A., El-Mleeh A., Hetta H.F., Al-Rejaie S., Alghamdi S., Zahoor M., Magdy Beshbishy A., Murata T., Zaragoza-Bastida A., et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium) Antibiotics. 2020;9:353. doi: 10.3390/antibiotics9060353. - DOI - PMC - PubMed
-
- Aati H.Y., Perveen S., Orfali R., Al-Taweel A.M., Aati S., Wanner J., Khan A., Mehmood R. Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia. Arab. J. Chem. 2020;13:8209–8217. doi: 10.1016/j.arabjc.2020.09.055. - DOI
-
- Bunse M., Daniels R., Gründemann C., Heilmann J., Kammerer D.R., Keusgen M., Lindequist U., Melzig M.F., Morlock G.E., Schulz H., et al. Essential oils as multicomponent mixtures and their potential for human health and well-being. Front. Pharmacol. 2022;13:956541. doi: 10.3389/fphar.2022.956541. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

