Identification and Expression Analysis of the Isopentenyl Transferase (IPT) Gene Family under Lack of Nitrogen Stress in Oilseed (Brassica napus L.)
- PMID: 37299144
- PMCID: PMC10255845
- DOI: 10.3390/plants12112166
Identification and Expression Analysis of the Isopentenyl Transferase (IPT) Gene Family under Lack of Nitrogen Stress in Oilseed (Brassica napus L.)
Abstract
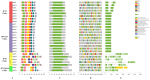
BnIPT gene family members in Brassica napus and analyzing their expression under different exogenous hormones and abiotic stress treatments to provide a theoretical basis for clarifying their functions and molecular genetic mechanisms in nitrogen deficiency stress tolerance of B. napus. Using the Arabidopsis IPT protein as the seed sequence, combined with the IPT protein domain PF01715, 26 members of the BnIPT gene family were identified from the whole genome of the rape variety ZS11. Additionally, the physicochemical properties and structures, phylogenetic relationships, synteny relationships, protein-protein interaction network, and gene ontology enrichment were analyzed. Based on transcriptome data, the expression patterns of the BnIPT gene under different exogenous hormone and abiotic stress treatments were analyzed. We used the qPCR method to identify the relative expression level of BnIPT genes that may be related to the stress resistance of rapeseed in transcriptome analysis under normal nitrogen (N: 6 mmol·L-1) and nitrogen deficiency (N: 0) conditions and analyzed its effect on rapeseed under nitrogen deficiency stress role in tolerance. In response to nitrogen deficiency signals, the BnIPT gene showed a trend of up-regulation in shoots and down-regulation in roots, indicating that it may affect the process of nitrogen transport and redistribution to enhance the stress resistance of rapeseed to respond to the nitrogen deficiency stress. This study provides a theoretical basis for clarifying the function and molecular genetic mechanism of the BnIPT gene family in nitrogen deficiency stress tolerance in rape.
Keywords: Brassica napus; IPT gene family; abiotic stress tolerance; nitrogen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures










Similar articles
-
Identification and expression analysis of the Xyloglucan transglycosylase/hydrolase (XTH) gene family under abiotic stress in oilseed (Brassica napus L.).BMC Plant Biol. 2024 May 15;24(1):400. doi: 10.1186/s12870-024-05121-5. BMC Plant Biol. 2024. PMID: 38745278 Free PMC article.
-
Identification and expression analysis of the cysteine synthase (CSase) gene family in Brassica napus L. under abiotic stress.BMC Plant Biol. 2025 Jun 5;25(1):770. doi: 10.1186/s12870-025-06532-8. BMC Plant Biol. 2025. PMID: 40474087 Free PMC article.
-
Comprehensive Identification and Expression Profiling of Epidermal Pattern Factor (EPF) Gene Family in Oilseed Rape (Brassica napus L.) under Salt Stress.Genes (Basel). 2024 Jul 12;15(7):912. doi: 10.3390/genes15070912. Genes (Basel). 2024. PMID: 39062691 Free PMC article.
-
Phylogenomic analysis of 20S proteasome gene family reveals stress-responsive patterns in rapeseed (Brassica napus L.).Front Plant Sci. 2022 Oct 31;13:1037206. doi: 10.3389/fpls.2022.1037206. eCollection 2022. Front Plant Sci. 2022. PMID: 36388569 Free PMC article.
-
Omics: The way forward to enhance abiotic stress tolerance in Brassica napus L.GM Crops Food. 2021 Jan 2;12(1):251-281. doi: 10.1080/21645698.2020.1859898. GM Crops Food. 2021. PMID: 33464960 Free PMC article. Review.
Cited by
-
Genome-wide investigation of cytokinin oxidase/dehydrogenase (CKX) family genes in Brassica juncea with an emphasis on yield-influencing CKX.Sci Rep. 2025 May 29;15(1):18825. doi: 10.1038/s41598-024-81004-x. Sci Rep. 2025. PMID: 40442160 Free PMC article.
-
Identification of GA2ox Family Genes and Expression Analysis under Gibberellin Treatment in Pineapple (Ananas comosus (L.) Merr.).Plants (Basel). 2023 Jul 17;12(14):2673. doi: 10.3390/plants12142673. Plants (Basel). 2023. PMID: 37514287 Free PMC article.
-
Genome-Wide Identification, Expression, and Protein Analysis of CKX and IPT Gene Families in Radish (Raphanus sativus L.) Reveal Their Involvement in Clubroot Resistance.Int J Mol Sci. 2024 Aug 17;25(16):8974. doi: 10.3390/ijms25168974. Int J Mol Sci. 2024. PMID: 39201660 Free PMC article.
-
Identification and Expression Profiling of the Cytokinin Synthesis Gene Family IPT in Maize.Genes (Basel). 2025 Mar 31;16(4):415. doi: 10.3390/genes16040415. Genes (Basel). 2025. PMID: 40282375 Free PMC article.
-
Identification of the Casparian strip integrity factor (CIF) gene family in Brassica napus and functional prediction of mature CIF small peptides.BMC Plant Biol. 2025 Jun 5;25(1):767. doi: 10.1186/s12870-025-06633-4. BMC Plant Biol. 2025. PMID: 40474073 Free PMC article.
References
-
- Mik V., Szucova L., Spichal L., Plihal O., Nisler J., Zahajska L., Dolezal K., Strnad M. N9-Substituted N(6)-[(3-methylbut-2-en-1-yl)amino]purine derivatives and their biological activity in selected cytokinin bioassays. Bioorg. Med. Chem. 2011;19:7244–7251. doi: 10.1016/j.bmc.2011.09.052. - DOI - PubMed
LinkOut - more resources
Full Text Sources

