Biomimetic Hydroxyapatite Crystals Growth on Phosphorylated Chitosan Films by In Vitro Mineralization Used as Dental Substitute Materials
- PMID: 37299269
- PMCID: PMC10255572
- DOI: 10.3390/polym15112470
Biomimetic Hydroxyapatite Crystals Growth on Phosphorylated Chitosan Films by In Vitro Mineralization Used as Dental Substitute Materials
Abstract
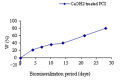
Chitosan (CS) films exhibit great potential as a substrate for the in vitro mineralization process. In this study, to mimic the formation of nanohydroxyapatite (HAP) as natural tissue, CS films coated with a porous calcium phosphate were investigated using scanning electron microscopy (SEM), Energy dispersive X-ray spectroscopy (EDX), Fourier transforms infrared spectroscopy (FTIR), X-ray diffractometry (XRD) and X-ray photoelectron spectroscopy (XPS). Calcium phosphate coating deposited on phosphorylated derivatives of CS was obtained by a process based on phosphorylation, Ca(OH)2 treatment and artificial saliva solution (ASS) immersion. The phosphorylated CS films (PCS) were obtained by partial hydrolysis of the PO4 functionalities. It was demonstrated that this precursor phase could induce the growth and the nucleation of the porous calcium phosphate coating when immersed in ASS. Moreover, oriented crystals and qualitative control of calcium phosphate phases on CS matrices are obtained in a biomimetic mode. Furthermore, in vitro antimicrobial activity of PCS was evaluated against three species of oral bacteria and fungi. It revealed an increase in antimicrobial activity with minimum inhibition concentration (MIC) values of 0.10% (Candida albicans), 0.05% (Staphylococcus aureus) and 0.025% (Escherichia coli) which proves their possible use as dental substitute materials.
Keywords: biomineralization; calcium phosphate; chitosan film; dental material; hydroxyapatite; phosphorylated chitosan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Porous calcium phosphate coating over phosphorylated chitosan film by a biomimetic method.Biomaterials. 1999 May;20(9):879-84. doi: 10.1016/s0142-9612(98)00243-9. Biomaterials. 1999. PMID: 10226713
-
Chitosan as a Templating Agent of Calcium Phosphate Crystalline Phases in Biomimetic Mineralization: Theoretical and Experimental Studies.ACS Appl Mater Interfaces. 2024 Nov 20;16(46):63155-63169. doi: 10.1021/acsami.4c11887. Epub 2024 Nov 11. ACS Appl Mater Interfaces. 2024. PMID: 39526983
-
Biomimetic chitosan-calcium phosphate composites with potential applications as bone substitutes: preparation and characterization.J Biomed Mater Res B Appl Biomater. 2012 Apr;100(3):700-8. doi: 10.1002/jbm.b.32502. Epub 2011 Nov 28. J Biomed Mater Res B Appl Biomater. 2012. PMID: 22121073
-
Mineralization of pristine chitosan film through biomimetic process.Int J Biol Macromol. 2011 Oct 1;49(3):385-9. doi: 10.1016/j.ijbiomac.2011.05.021. Epub 2011 May 27. Int J Biol Macromol. 2011. PMID: 21641923
-
Characterization of biomimetic calcium phosphate on phosphorylated chitosan films.J Biomed Mater Res A. 2007 Aug;82(2):343-53. doi: 10.1002/jbm.a.31070. J Biomed Mater Res A. 2007. PMID: 17295230
Cited by
-
Polycarboxy/Sulfo Betaine-Calcium Phosphate Hybrid Materials with a Remineralization Potential.Materials (Basel). 2023 Oct 11;16(20):6640. doi: 10.3390/ma16206640. Materials (Basel). 2023. PMID: 37895622 Free PMC article.
-
Biomineralization of dental tissues with natural drugs: a comprehensive review.Saudi Dent J. 2025 Jul 15;37(4-6):29. doi: 10.1007/s44445-025-00036-9. Saudi Dent J. 2025. PMID: 40663199 Free PMC article. Review.
-
Potency of the Combination of Chitosan and Hydroxyapatite on Angiogenesis and Fibroblast Cell Proliferation in Direct Pulp Capping of Rattus norvegicus.Eur J Dent. 2024 Oct;18(4):1135-1141. doi: 10.1055/s-0044-1782212. Epub 2024 May 2. Eur J Dent. 2024. PMID: 38698616 Free PMC article.
-
Effect of nano-hydroxyapatite filling on masticatory function and gingival sulcular fluid inflammatory factor levels in periapical inflammation.Biomed Eng Online. 2025 May 21;24(1):63. doi: 10.1186/s12938-025-01374-9. Biomed Eng Online. 2025. PMID: 40399943 Free PMC article.
-
Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates.Polymers (Basel). 2024 Jan 10;16(2):206. doi: 10.3390/polym16020206. Polymers (Basel). 2024. PMID: 38257005 Free PMC article. Review.
References
-
- Le Geros R.Z. Apatite in biological systems. Prog Cryst. Growth Charact. 1981;4:1–45. doi: 10.1016/0146-3535(81)90046-0. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

