Iron Complexes with Antarctic Krill-Derived Peptides Show Superior Effectiveness to Their Original Protein-Iron Complexes in Mice with Iron Deficiency Anemia
- PMID: 37299473
- PMCID: PMC10255654
- DOI: 10.3390/nu15112510
Iron Complexes with Antarctic Krill-Derived Peptides Show Superior Effectiveness to Their Original Protein-Iron Complexes in Mice with Iron Deficiency Anemia
Abstract
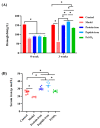
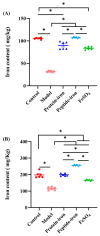
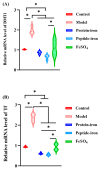
Antarctic krill protein-iron complex and peptide-iron complex were acquired to investigate their iron bioavailability, expression of iron-regulated genes, and in vivo antioxidant capacity. Results indicated that the Antarctic krill peptide-iron complex significantly increased the hemoglobin (Hb), serum iron (SI), and iron contents in the liver and spleen in iron-deficiency anemia (IDA) mice (p < 0.05) compared with those of the Antarctic krill protein-iron complex. Despite the gene expressions of the divalent metal transporter 1(DMT1), the transferrin (Tf), and the transferrin receptor (TfR) being better regulated by both Antarctic krill peptide-iron complex and protein-iron complex, the relative iron bioavailability of the Antarctic krill peptide-iron complex group (152.53 ± 21.05%) was significantly higher than that of the protein-iron complex group (112.75 ± 9.60%) (p < 0.05). Moreover, Antarctic krill peptide-iron complex could enhance the antioxidant enzyme activities of superoxidase dismutase (SOD) and glutathione peroxidase (GSH-Px), reduce the malondialdehyde (MDA) level in IDA mice compared with the protein-iron complex, and reduce the cell damage caused by IDA. Therefore, these results indicated that Antarctic krill peptide-iron complex could be used as a highly efficient and multifunctional iron supplement.
Keywords: Antarctic krill peptide–iron; IDA mice; in vivo antioxidant capacity; iron bioavailability; iron-regulated genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Molecular Mechanisms of Iron Transport and Homeostasis Regulated by Antarctic Krill-Derived Heptapeptide-Iron Complex.J Agric Food Chem. 2024 Apr 3;72(13):7517-7532. doi: 10.1021/acs.jafc.3c05812. Epub 2024 Mar 25. J Agric Food Chem. 2024. PMID: 38527166
-
Antarctic Krill Protein Amyloid Fibrils as a Novel Iron Carrier for the Improvement of Iron Deficiency.J Agric Food Chem. 2025 Feb 5;73(5):3170-3180. doi: 10.1021/acs.jafc.4c11046. Epub 2025 Jan 24. J Agric Food Chem. 2025. PMID: 39851240
-
The Protective Effect of Antarctic Krill Oil on Cognitive Function by Inhibiting Oxidative Stress in the Brain of Senescence-Accelerated Prone Mouse Strain 8 (SAMP8) Mice.J Food Sci. 2018 Feb;83(2):543-551. doi: 10.1111/1750-3841.14044. Epub 2018 Jan 19. J Food Sci. 2018. PMID: 29350764
-
The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress.Free Radic Biol Med. 2013 Dec;65:1174-1194. doi: 10.1016/j.freeradbiomed.2013.09.001. Epub 2013 Sep 12. Free Radic Biol Med. 2013. PMID: 24036104 Review.
-
Reproductive traits of common Euphausids in the south of Antarctic Polar Front.Ying Yong Sheng Tai Xue Bao. 2021 Nov 15;32(11):4156-4164. doi: 10.13287/j.1001-9332.202111.029. Ying Yong Sheng Tai Xue Bao. 2021. PMID: 34898132 Review. English.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

