One-Step Hydrothermal Synthesis of Cu2ZnSnS4 Nanoparticles as an Efficient Visible Light Photocatalyst for the Degradation of Congo Red Azo Dye
- PMID: 37299634
- PMCID: PMC10254566
- DOI: 10.3390/nano13111731
One-Step Hydrothermal Synthesis of Cu2ZnSnS4 Nanoparticles as an Efficient Visible Light Photocatalyst for the Degradation of Congo Red Azo Dye
Abstract
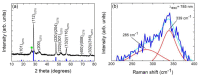
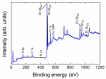
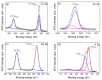
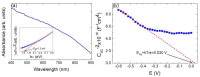
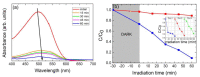
A hydrothermal method was successfully employed to synthesize kesterite Cu2ZnSnS4 (CZTS) nanoparticles. X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), field-emission scanning electron microscopy (FE-SEM), energy-dispersive X-ray spectroscopy (EDS), transmission electron microscopy (TEM), and optical ultraviolet-visible (UV-vis) spectroscopy were used for characterization of structural, chemical, morphological, and optical properties. XRD results confirmed that a nanocrystalline CZTS phase corresponding to the kesterite structure was formed. Raman analysis confirmed the existence of single pure phase CZTS. XPS results revealed the oxidation states as Cu+, Zn2+, Sn4+, and S2-. FESEM and TEM micrograph images revealed the presence of nanoparticles with average sizes between 7 nm to 60 nm. The synthesized CZTS nanoparticles bandgap was found to be 1.5 eV which is optimal for solar photocatalytic degradation applications. The properties as a semiconductor material were evaluated through the Mott-Schottky analysis. The photocatalytic activity of CZTS has been investigated through photodegradation of Congo red azo dye solution under solar simulation light irradiation, proving to be an excellent photo-catalyst for CR where 90.2% degradation could be achieved in just 60 min. Furthermore, the prepared CZTS was reusable and can be repeatedly used to remove Congo red dye from aqueous solutions.
Keywords: CZTS; Congo red azo dye; hydrothermal; photocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wang A., He M., Green M.A., Sun K., Hao X. A Critical Review on the Progress of Kesterite Solar Cells: Current Strategies and Insights. Adv. Energy Mater. 2023;13:2203046. doi: 10.1002/aenm.202203046. - DOI
-
- Zhao Y., Yu Z., Hu J., Zheng Z., Ma H., Sun K., Hao X., Liang G., Fan P., Zhang X., et al. Over 12% efficient kesterite solar cell via back interface engineering. J. Energy Chem. 2022;75:321–329. doi: 10.1016/j.jechem.2022.08.031. - DOI
-
- Altaf C.T., Abdullayeva N., Sankir N.D., Sankir N.D. Photoelectrochemical Solar Cells. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2018. Copper-Based Chalcopyrite and Kesterite Materials for Solar Hydrogen Generation; pp. 251–303.
LinkOut - more resources
Full Text Sources

