BMP7 expression in mammalian cortical radial glial cells increases the length of the neurogenic period
- PMID: 37300483
- PMCID: PMC10762677
- DOI: 10.1093/procel/pwad036
BMP7 expression in mammalian cortical radial glial cells increases the length of the neurogenic period
Abstract
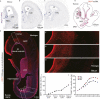
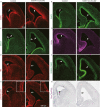
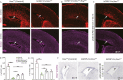
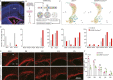
The seat of human intelligence is the human cerebral cortex, which is responsible for our exceptional cognitive abilities. Identifying principles that lead to the development of the large-sized human cerebral cortex will shed light on what makes the human brain and species so special. The remarkable increase in the number of human cortical pyramidal neurons and the size of the human cerebral cortex is mainly because human cortical radial glial cells, primary neural stem cells in the cortex, generate cortical pyramidal neurons for more than 130 days, whereas the same process takes only about 7 days in mice. The molecular mechanisms underlying this difference are largely unknown. Here, we found that bone morphogenic protein 7 (BMP7) is expressed by increasing the number of cortical radial glial cells during mammalian evolution (mouse, ferret, monkey, and human). BMP7 expression in cortical radial glial cells promotes neurogenesis, inhibits gliogenesis, and thereby increases the length of the neurogenic period, whereas Sonic Hedgehog (SHH) signaling promotes cortical gliogenesis. We demonstrate that BMP7 signaling and SHH signaling mutually inhibit each other through regulation of GLI3 repressor formation. We propose that BMP7 drives the evolutionary expansion of the mammalian cortex by increasing the length of the neurogenic period.
Keywords: BMP7; SHH; cortical evolution; cortical gliogenesis; cortical neurogenesis; radial glia.
©The Author(s) 2023. Published by Oxford University Press on behalf of Higher Education Press.
Conflict of interest statement
None declared.
Figures



References
-
- Abu-Khalil A, Fu L, Grove EAet al. . Wnt genes define distinct boundaries in the developing human brain: implications for human forebrain patterning. J Comp Neurol 2004;474:276–288. - PubMed
-
- Aoto K, Nishimura T, Eto Ket al. . Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol 2002;251:320–332. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

