The experience of neoadjuvant chemotherapy versus upfront surgery in resectable pancreatic cancer: a cross sectional study
- PMID: 37300888
- PMCID: PMC10498854
- DOI: 10.1097/JS9.0000000000000495
The experience of neoadjuvant chemotherapy versus upfront surgery in resectable pancreatic cancer: a cross sectional study
Abstract
Background: Upfront resection (UR) followed by adjuvant chemotherapy remains the standard treatment for resectable pancreatic cancer. There is increasing evidence suggesting favourable outcomes toward neoadjuvant chemotherapy (NAC) followed by surgery.
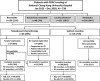
Methods: All clinical staging with resectable pancreatic cancer patients treated at a tertiary medical centre from 2013 to 2020 were identified. The baseline characteristics, treatment course, surgery outcome and survival results of UR or NAC were compared.
Results: Finally, in 159 resectable patients, 46 patients (29%) underwent NAC and 113 patients (71%) received UR. In NAC, 11 patients (24%) did not receive resection, 4 (36.4%) for comorbidity, 2 (18.2%) for patient refusal and 2 (18.2%) for disease progression. In UR, 13 patients (12%) were unresectable intraoperatively; 6 (46.2%) for locally advanced and 5 (38.5%) for distant metastasis. Overall, 97% of patients in NAC and 58% of patients in UR completed adjuvant chemotherapy. As of data cut-off, 24 patients (69%) in NAC and 42 patients (29%) in UR were still tumour free. The median recurrence-free survival in NAC, UR with adjuvant chemotherapy and without adjuvant chemotherapy were 31.3 months (95% CI, 14.4-not estimable), 10.6 months (95% CI, 9.0-14.3) and 8.5 months (95% CI, 5.8-11.8), P =0.036; and the median overall survival in each group were not reached (95% CI, 29.7-not estimable), 25.9 months (95% CI, 21.1-40.5) and 21.7 months (12.0-32.8), P =0.0053. Based on initial clinical staging, the median overall survival of NAC was not significantly different from UR with a tumour less than or equal to 2 cm, P =0.29. NAC patients had a higher R0 resection rate (83% versus 53%), lower recurrence rate (31% versus 71%) and harvested median number lymph node (23 versus 15).
Conclusion: This study demonstrates that NAC is superior to UR in resectable pancreatic cancer with better survival.
Trial registration: ClinicalTrials.gov NCT05700188.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
All authors have no conflicts of interest.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



Comment in
-
A commentary on 'The experience of neoadjuvant chemotherapy versus upfront surgery in resectable pancreatic cancer: a cross sectional study'.Int J Surg. 2023 Oct 1;109(10):3209-3210. doi: 10.1097/JS9.0000000000000588. Int J Surg. 2023. PMID: 37402310 Free PMC article. No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL, et al. . Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Mizrahi JD, Surana R, Valle JW, et al. . Pancreatic cancer. Lancet 2020;395:2008–2020. - PubMed
-
- Conroy T, Hammel P, Hebbar M, et al. . FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 2018;379:2395–2406. - PubMed
-
- Snyder RA, Parikh AA. Actual survival in patients with resected pancreatic cancer: how do real-world data compare with clinical trial evidence? Ann Surg Oncol 2021;28:8014–8016. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

