Targeting cytokine-like protein FAM3D lowers blood pressure in hypertension
- PMID: 37301198
- PMCID: PMC10313939
- DOI: 10.1016/j.xcrm.2023.101072
Targeting cytokine-like protein FAM3D lowers blood pressure in hypertension
Abstract
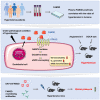
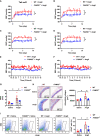
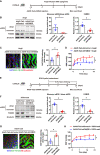
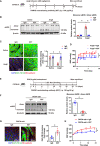
Current antihypertensive options still incompletely control blood pressure, suggesting the existence of uncovered pathogenic mechanisms. Here, whether cytokine-like protein family with sequence similarity 3, member D (FAM3D) is involved in hypertension etiology is evaluated. A case-control study exhibits that FAM3D is elevated in patients with hypertension, with a positive association with odds of hypertension. FAM3D deficiency significantly ameliorates angiotensin II (AngII)-induced hypertension in mice. Mechanistically, FAM3D directly causes endothelial nitric oxide synthase (eNOS) uncoupling and impairs endothelium-dependent vasorelaxation, whereas 2,4-diamino-6-hydroxypyrimidine to induce eNOS uncoupling abolishes the protective effect of FAM3D deficiency against AngII-induced hypertension. Furthermore, antagonism of formyl peptide receptor 1 (FPR1) and FPR2 or the suppression of oxidative stress blunts FAM3D-induced eNOS uncoupling. Translationally, targeting endothelial FAM3D by adeno-associated virus or intraperitoneal injection of FAM3D-neutralizing antibodies markedly ameliorates AngII- or deoxycorticosterone acetate (DOCA)-salt-induced hypertension. Conclusively, FAM3D causes eNOS uncoupling through FPR1- and FPR2-mediated oxidative stress, thereby exacerbating the development of hypertension. FAM3D may be a potential therapeutic target for hypertension.
Keywords: FAM3D; antihypertensive treatment; endothelial dysfunction; hypertension.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Oparil S., Schmieder R.E. New approaches in the treatment of hypertension. Circ. Res. 2015;116:1074–1095. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

