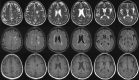
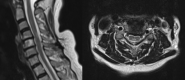
Half-dose ocrelizumab in selected patients with relapsing-remitting multiple sclerosis
- PMID: 37301802
- PMCID: PMC10257484
- DOI: 10.1007/s13760-023-02303-0
Half-dose ocrelizumab in selected patients with relapsing-remitting multiple sclerosis
Keywords: Half-dose; Multiple sclerosis; Ocrelizumab; Personalized.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Serum sickness in a multiple sclerosis patient treated with ocrelizumab.J Neurol. 2024 Feb;271(2):727-728. doi: 10.1007/s00415-023-12082-6. Epub 2023 Oct 31. J Neurol. 2024. PMID: 37907726 No abstract available.
-
Two cases of meningitis associated with ocrelizumab therapy.Mult Scler Relat Disord. 2020 Feb;38:101866. doi: 10.1016/j.msard.2019.101866. Epub 2019 Nov 27. Mult Scler Relat Disord. 2020. PMID: 31821962
-
Ocrelizumab (Ocrevus) for MS.Med Lett Drugs Ther. 2017 Jun 19;59(1523):98-101. Med Lett Drugs Ther. 2017. PMID: 28609424 No abstract available.
-
Ocrelizumab-associated cryptogenic organizing pneumonia in multiple sclerosis: Two case reports and comprehensive literature review.Mult Scler. 2025 May;31(6):740-743. doi: 10.1177/13524585241295677. Epub 2024 Nov 21. Mult Scler. 2025. PMID: 39569527 Review.
-
Ocrelizumab for the treatment of multiple sclerosis.Expert Rev Neurother. 2019 Feb;19(2):97-108. doi: 10.1080/14737175.2019.1561284. Epub 2018 Dec 28. Expert Rev Neurother. 2019. PMID: 30570368 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical