Effects of risperidone/paliperidone versus placebo on cognitive functioning over the first 6 months of treatment for psychotic disorder: secondary analysis of a triple-blind randomised clinical trial
- PMID: 37301832
- PMCID: PMC10257667
- DOI: 10.1038/s41398-023-02501-7
Effects of risperidone/paliperidone versus placebo on cognitive functioning over the first 6 months of treatment for psychotic disorder: secondary analysis of a triple-blind randomised clinical trial
Abstract
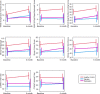
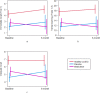
The drivers of cognitive change following first-episode psychosis remain poorly understood. Evidence regarding the role of antipsychotic medication is primarily based on naturalistic studies or clinical trials without a placebo arm, making it difficult to disentangle illness from medication effects. A secondary analysis of a randomised, triple-blind, placebo-controlled trial, where antipsychotic-naive patients with first-episode psychotic disorder were allocated to receive risperidone/paliperidone or matched placebo plus intensive psychosocial therapy for 6 months was conducted. A healthy control group was also recruited. A cognitive battery was administered at baseline and 6 months. Intention-to-treat analysis involved 76 patients (antipsychotic medication group: 37; 18.6Mage [2.9] years; 21 women; placebo group: 39; 18.3Mage [2.7]; 22 women); and 42 healthy controls (19.2Mage [3.0] years; 28 women). Cognitive performance predominantly remained stable (working memory, verbal fluency) or improved (attention, processing speed, cognitive control), with no group-by-time interaction evident. However, a significant group-by-time interaction was observed for immediate recall (p = 0.023), verbal learning (p = 0.024) and delayed recall (p = 0.005). The medication group declined whereas the placebo group improved on each measure (immediate recall: p = 0.024; ηp2 = 0.062; verbal learning: p = 0.015; ηp2 = 0.072 both medium effects; delayed recall: p = 0.001; ηp2 = 0.123 large effect). The rate of change for the placebo and healthy control groups was similar. Per protocol analysis (placebo n = 16, medication n = 11) produced similar findings. Risperidone/paliperidone may worsen verbal learning and memory in the early months of psychosis treatment. Replication of this finding and examination of various antipsychotic agents are needed in confirmatory trials. Antipsychotic effects should be considered in longitudinal studies of cognition in psychosis.Trial registration: Australian New Zealand Clinical Trials Registry ( http://www.anzctr.org.au/ ; ACTRN12607000608460).
© 2023. The Author(s).
Conflict of interest statement
KA was supported by a NHMRC Career Development Fellowship (1141207) and a University of Melbourne Dame Kate Campbell Fellowship. KA has received funding from the NHMRC, MRFF and Wellcome Trust—all unrelated to this work. MB is supported by a NHMRC Senior Principal Research Fellowship and Leadership 3 Investigator grant (1156072 and 2017131). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck and Merck and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier—all unrelated to this work. ADT has received honoraria for lectures for Otsuka Pty, Janssen and Janssen and Servier and has served on an advisory board for Servier—all unrelated to this work. BN is supported by a NHMRC Senior Research Fellowship (1137687) and a University of Melbourne Dame Kate Campbell Fellowship—all unrelated to this work. MA-J was supported by an Investigator Grant (APP1177235) from the NHMRC and a Dame Kate Campbell Fellowship from The University of Melbourne—all unrelated to this work. CP was supported by a NHMRC Senior Principal Research Fellowship (1105825), and NHMRC L3 Investigator Grant (1196508). Over the past three years, CP has received funding from NHMRC, Lundbeck Foundation, MRFF, and has received honoraria for talks at educational meetings and as a member of an advisory board for Lundbeck, Australia Pty Ltd—all unrelated to this work. The other authors report no biomedical financial interests or potential conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

