Unsaturated fatty acids uniquely alter aggregation rate of α-synuclein and insulin and change the secondary structure and toxicity of amyloid aggregates formed in their presence
- PMID: 37302013
- PMCID: PMC10405295
- DOI: 10.1096/fj.202300003R
Unsaturated fatty acids uniquely alter aggregation rate of α-synuclein and insulin and change the secondary structure and toxicity of amyloid aggregates formed in their presence
Abstract
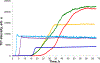
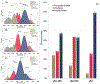
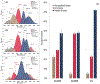
Docosahexaenoic (DHA) and arachidonic acids (ARA) are omega-3 and omega-6 long-chain polyunsaturated fatty acids (LCPUFAs). These molecules constitute a substantial portion of phospholipids in plasma membranes. Therefore, both DHA and ARA are essential diet components. Once consumed, DHA and ARA can interact with a large variety of biomolecules, including proteins such as insulin and α-synuclein (α-Syn). Under pathological conditions known as injection amyloidosis and Parkinson's disease, these proteins aggregate forming amyloid oligomers and fibrils, toxic species that exert high cell toxicity. In this study, we investigate the role of DHA and ARA in the aggregation properties of α-Syn and insulin. We found that the presence of both DHA and ARA at the equimolar concentrations strongly accelerated aggregation rates of α-Syn and insulin. Furthermore, LCPUFAs substantially altered the secondary structure of protein aggregates, whereas no noticeable changes in the fibril morphology were observed. Nanoscale Infrared analysis of α-Syn and insulin fibrils grown in the presence of both DHA and ARA revealed the presence of LCPUFAs in these aggregates. We also found that such LCPUFAs-rich α-Syn and insulin fibrils exerted significantly greater toxicities compared to the aggregates grown in the LCPUFAs-free environment. These findings show that interactions between amyloid-associated proteins and LCPUFAs can be the underlying molecular cause of neurodegenerative diseases.
Keywords: AFM-IR; LDH; ROS; alpha-synuclein; arachidonic acid; docosahexaenoic acid; insulin; unsaturated farry acids.
© 2023 Federation of American Societies for Experimental Biology.
Conflict of interest statement
DISCLOSURES
The authors declare no competing financial interests.
Figures



Similar articles
-
Long-Chain Polyunsaturated Fatty Acids Accelerate the Rate of Insulin Aggregation and Enhance Toxicity of Insulin Aggregates.ACS Chem Neurosci. 2024 Jan 3;15(1):147-154. doi: 10.1021/acschemneuro.3c00583. Epub 2023 Dec 21. ACS Chem Neurosci. 2024. PMID: 38127718 Free PMC article.
-
The influence of zwitterionic and anionic phospholipids on protein aggregation.Biophys Chem. 2024 Mar;306:107174. doi: 10.1016/j.bpc.2024.107174. Epub 2024 Jan 7. Biophys Chem. 2024. PMID: 38211368 Free PMC article. Review.
-
Elucidating the Role of Lipids in the Aggregation of Amyloidogenic Proteins.Acc Chem Res. 2023 Nov 7;56(21):2898-2906. doi: 10.1021/acs.accounts.3c00386. Epub 2023 Oct 12. Acc Chem Res. 2023. PMID: 37824095 Free PMC article.
-
Nanoscale imaging of individual amyloid aggregates extracted from brains of Alzheimer and Parkinson patients reveals presence of lipids in α-synuclein but not in amyloid β1-42 fibrils.Protein Sci. 2023 Apr;32(4):e4598. doi: 10.1002/pro.4598. Protein Sci. 2023. PMID: 36823759 Free PMC article.
-
Nitroalkylation of α-Synuclein by Nitro-Oleic Acid: Implications for Parkinson's Disease.Adv Exp Med Biol. 2019;1127:169-179. doi: 10.1007/978-3-030-11488-6_11. Adv Exp Med Biol. 2019. PMID: 31140178 Review.
Cited by
-
Nano-Infrared Detection and Identification of Bacteria at the Single-Cell Level.Anal Chem. 2025 May 6;97(17):9535-9539. doi: 10.1021/acs.analchem.5c01677. Epub 2025 Apr 21. Anal Chem. 2025. PMID: 40258302 Free PMC article.
-
Elucidation of cytotoxicity of α-Synuclein fibrils on immune cells.Biochim Biophys Acta Proteins Proteom. 2025 Feb 1;1873(2):141061. doi: 10.1016/j.bbapap.2024.141061. Epub 2024 Dec 16. Biochim Biophys Acta Proteins Proteom. 2025. PMID: 39694308 Free PMC article.
-
Lysophosphatidylcholine promoting α-Synuclein aggregation in Parkinson's disease: disrupting GCase glycosylation and lysosomal α-Synuclein degradation.NPJ Parkinsons Dis. 2025 Mar 15;11(1):47. doi: 10.1038/s41531-025-00902-7. NPJ Parkinsons Dis. 2025. PMID: 40089519 Free PMC article.
-
Long-Chain Polyunsaturated Fatty Acids Accelerate the Rate of Insulin Aggregation and Enhance Toxicity of Insulin Aggregates.ACS Chem Neurosci. 2024 Jan 3;15(1):147-154. doi: 10.1021/acschemneuro.3c00583. Epub 2023 Dec 21. ACS Chem Neurosci. 2024. PMID: 38127718 Free PMC article.
-
The influence of zwitterionic and anionic phospholipids on protein aggregation.Biophys Chem. 2024 Mar;306:107174. doi: 10.1016/j.bpc.2024.107174. Epub 2024 Jan 7. Biophys Chem. 2024. PMID: 38211368 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

