White Matter Hyperintensity Volume and Amyloid-PET Synergistically Impact Memory Independent of Tau-PET in Older Adults Without Dementia
- PMID: 37302031
- PMCID: PMC10357163
- DOI: 10.3233/JAD-221209
White Matter Hyperintensity Volume and Amyloid-PET Synergistically Impact Memory Independent of Tau-PET in Older Adults Without Dementia
Abstract
Background: Alzheimer's disease (AD) and cerebrovascular disease are common, co-existing pathologies in older adults. Whether the effects of cerebrovascular disease and AD biomarkers on cognition are additive or synergistic remains unclear.
Objective: To examine whether white matter hyperintensity (WMH) volume moderates the independent association between each AD biomarker and cognition.
Methods: In 586 older adults without dementia, linear regressions tested the interaction between amyloid-β (Aβ) positron emission tomography (PET) and WMH volume on cognition, independent of tau-PET. We also tested the interaction between tau-PET and WMH volume on cognition, independent of Aβ-PET.
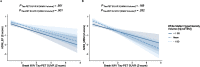
Results: Adjusting for tau-PET, the quadratic effect of WMH interacted with Aβ-PET to impact memory. There was no interaction between either the linear or quadratic effect of WMH and Aβ-PET on executive function. There was no interaction between WMH volume and tau-PET on either cognitive measure.
Conclusion: Results suggest that cerebrovascular lesions act synergistically with Aβ to affect memory, independent of tau, highlighting the importance of incorporating vascular pathology into biomarker assessment of AD.
Keywords: Alzheimer’s disease; amyloid-β; executive function; memory; tau; white matter hyperintensities.
Conflict of interest statement
Dr. Bondi is a paid consultant for Eisai, Novartis and Roche Pharmaceuticals and receives royalties from Oxford University Press.
Dr. Thomas, Dr. Nation, Dr. Bondi, and Dr. Bangen are Editorial Board members of the Journal of Alzheimer’s Disease and were not involved in peer reviewing the present paper.
No other authors have competing interests to declare.
Figures

References
-
- McKhann, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. - PubMed
-
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Tredici KD, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 71, 362–381. - PMC - PubMed
-
- Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, Ayakta N, Cantwell A, Janabi M, Lauriola M, O’Neil JP, Gorno-Tempini ML, Miller ZA, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD (2017) Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 140, 3286–3300. - PMC - PubMed
-
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD (2016) Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139, 1551–1567. - PMC - PubMed

