Parkinson's Disease Drug Therapies in the Clinical Trial Pipeline: 2023 Update
- PMID: 37302040
- PMCID: PMC10357160
- DOI: 10.3233/JPD-239901
Parkinson's Disease Drug Therapies in the Clinical Trial Pipeline: 2023 Update
Abstract
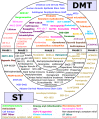
Background: Since 2020, annual reports on the clinical development of new drug-based therapies for the neurodegenerative condition of Parkinson's disease (PD) have been generated. These reviews have followed the progress of both "symptomatic treatments" (ST - improves/reduces symptoms of the condition) and "disease modifying treatments" (DMT - attempts to delay/slow progression by addressing the underlying biology of PD). Additional efforts have been made to further categorize these experimental treatments based on their mechanisms of action and class of drug.
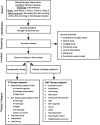
Methods: A dataset of clinical trials for drug therapies in PD was obtained using trial data downloaded from the ClinicalTrials.gov online registry. A breakdown analysis of all the studies that were active as of January 31st, 2023, was conducted.
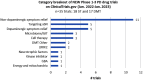
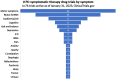
Results: There was a total of 139 clinical trials registered on the ClinicalTrials.gov website as active (with 35 trials newly registered since our last report). Of these trials, 76 (55%) were considered ST and 63 (45%) were designated DMT. Similar to previous years, approximately a third of the studies were in Phase 1 (n = 47; 34%), half (n = 72, 52%) were in Phase 2 and there were 20 (14%) studies in Phase 3. Novel therapies again represented the most dominant group of experimental treatments in this year's report with 58 (42%) trials testing new agents. Repurposed drugs are present in a third (n = 49, 35%) of trials, with reformulations and new claims representing 19% and 4% of studies, respectively.
Conclusions: Our fourth annual review of active clinical trials evaluating ST and DMT therapeutics for PD demonstrates that the drug development pipeline is dynamic and evolving. The slow progress and lack of agents transitioning from Phase 2 to Phase 3 is concerning, but collective efforts by various stakeholders are being made to accelerate the clinical trial process, with the aim of bringing new therapies to the PD community sooner.
Keywords: Clinical trials; Parkinson’s; disease modification; gene therapy; immunotherapy; inflammation; neuroprotection; studies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- World Health Organization technical brief “Parkinson disease: Apublic health approach”; ISBN: 9789240050983; https://www.who.int/publications/i/item/9789240050983.
-
- Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LMA, Hutten SJ, Sherer T, Marek K, Soto C; Parkinson’s Progression Markers Initiative (2023) Assessmentof heterogeneity among participants in the Parkinson’s ProgressionMarkers Initiative cohort using α-synuclein seedamplification: A cross-sectional study. Lancet Neurol 22(5), 407–417. - PMC - PubMed
-
- Path to Prevention Platform Trial; https://www.ppmi-info.org/study-design/path-to-prevention-platform-trial.
-
- Foltynie T, Gandhi S, Gonzalez-Robles C, Zeissler ML, Mills G, Barker R, Carpenter J, Schrag A, Schapira A, Bandmann O, Mullin S, Duffen J, McFarthing K, Chataway J, Parmar M, Carroll C; EJS ACT-PDConsortium (2023) Towards a multi-arm multi-stage platform trial of disease modifying approaches in Parkinson’s disease. Brain. Online ahead of print 10.1093/brain/awad063. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

