Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial
- PMID: 37302406
- PMCID: PMC11259948
- DOI: 10.1016/S1473-3099(23)00299-2
Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial
Erratum in
-
Correction to Lancet Infect Dis 2023; published online June 8. https://doi.org/10.1016/S1473-3099(23)00299-2.Lancet Infect Dis. 2023 Oct;23(10):e400. doi: 10.1016/S1473-3099(23)00562-5. Epub 2023 Sep 1. Lancet Infect Dis. 2023. PMID: 37666258 No abstract available.
Abstract
Background: Post-COVID-19 condition (also known as long COVID) is an emerging chronic illness potentially affecting millions of people. We aimed to evaluate whether outpatient COVID-19 treatment with metformin, ivermectin, or fluvoxamine soon after SARS-CoV-2 infection could reduce the risk of long COVID.
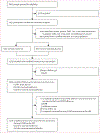
Methods: We conducted a decentralised, randomised, quadruple-blind, parallel-group, phase 3 trial (COVID-OUT) at six sites in the USA. We included adults aged 30-85 years with overweight or obesity who had COVID-19 symptoms for fewer than 7 days and a documented SARS-CoV-2 positive PCR or antigen test within 3 days before enrolment. Participants were randomly assigned via 2 × 3 parallel factorial randomisation (1:1:1:1:1:1) to receive metformin plus ivermectin, metformin plus fluvoxamine, metformin plus placebo, ivermectin plus placebo, fluvoxamine plus placebo, or placebo plus placebo. Participants, investigators, care providers, and outcomes assessors were masked to study group assignment. The primary outcome was severe COVID-19 by day 14, and those data have been published previously. Because the trial was delivered remotely nationwide, the a priori primary sample was a modified intention-to-treat sample, meaning that participants who did not receive any dose of study treatment were excluded. Long COVID diagnosis by a medical provider was a prespecified, long-term secondary outcome. This trial is complete and is registered with ClinicalTrials.gov, NCT04510194.
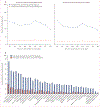
Findings: Between Dec 30, 2020, and Jan 28, 2022, 6602 people were assessed for eligibility and 1431 were enrolled and randomly assigned. Of 1323 participants who received a dose of study treatment and were included in the modified intention-to-treat population, 1126 consented for long-term follow-up and completed at least one survey after the assessment for long COVID at day 180 (564 received metformin and 562 received matched placebo; a subset of participants in the metformin vs placebo trial were also randomly assigned to receive ivermectin or fluvoxamine). 1074 (95%) of 1126 participants completed at least 9 months of follow-up. 632 (56·1%) of 1126 participants were female and 494 (43·9%) were male; 44 (7·0%) of 632 women were pregnant. The median age was 45 years (IQR 37-54) and median BMI was 29·8 kg/m2 (IQR 27·0-34·2). Overall, 93 (8·3%) of 1126 participants reported receipt of a long COVID diagnosis by day 300. The cumulative incidence of long COVID by day 300 was 6·3% (95% CI 4·2-8·2) in participants who received metformin and 10·4% (7·8-12·9) in those who received identical metformin placebo (hazard ratio [HR] 0·59, 95% CI 0·39-0·89; p=0·012). The metformin beneficial effect was consistent across prespecified subgroups. When metformin was started within 3 days of symptom onset, the HR was 0·37 (95% CI 0·15-0·95). There was no effect on cumulative incidence of long COVID with ivermectin (HR 0·99, 95% CI 0·59-1·64) or fluvoxamine (1·36, 0·78-2·34) compared with placebo.
Interpretation: Outpatient treatment with metformin reduced long COVID incidence by about 41%, with an absolute reduction of 4·1%, compared with placebo. Metformin has clinical benefits when used as outpatient treatment for COVID-19 and is globally available, low-cost, and safe.
Funding: Parsemus Foundation; Rainwater Charitable Foundation; Fast Grants; UnitedHealth Group Foundation; National Institute of Diabetes, Digestive and Kidney Diseases; National Institutes of Health; and National Center for Advancing Translational Sciences.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CTB was supported by grants (KL2TR002492 and UL1TR002494) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and by a grant (K23 DK124654–01-A1) from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. JBB was supported by grants (UL1TR002489 and UM1TR004406) from NCATS and reports contracted fees and travel support for contracted activities for consulting work paid to the University of North Carolina by Novo Nordisk; grant support by Dexcom, NovaTarg, Novo Nordisk, Sanofi, Tolerion, and vTv Therapeutics; personal compensation for consultation from Alkahest, Altimmune, Anji, AstraZeneca, Bayer, Biomea Fusion, Boehringer Ingelheim, CeQur, Cirius Therapeutics, Corcept Therapeutics, Eli Lilly, Fortress Biotech, GentiBio, Glycadia, Glyscend, Janssen, MannKind, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, Sanofi, Stability Health, Terns, Valo, and Zealand Pharma; and stock or stock options in Glyscend, Mellitus Health, Pendulum Therapeutics, PhaseBio, Praetego, and Stability Health. JMN was supported by a grant (K23HL133604) from the National Heart, Lung, and Blood Institute of the NIH. DJO was supported by the Institute for Engineering in Medicine, University of Minnesota Office of Academic and Clinical Affairs COVID-19 Rapid Response Grant, the Earl E Bakken Professorship for Engineering in Medicine, and by grants (U54 CA210190 and P01 CA254849) from the National Cancer Institute of the NIH. TAM was supported in part by the Medtronic Faculty Fellowship. DML receives funding from NIH RECOVER (OT2HL161847). LKS was supported by NIH grants (18X107CF6 and 18X107CF5) through a contract with Leidos Biomedical and by grants from the National Heart, Lung, and Blood Institute of the NIH (T32HL129956), and the NIH (R01LM012982 and R21LM012744). MAP receives grants from the Bill & Melinda Gates Foundation (INV-017069), Minnesota Partnership for Biotechnology and Medical Genomics (00086722), and the National Heart, Lung, and Blood Institute of the NIH (OT2HL156812); and consulting fees from Opticyte and Cytovale. All other authors declare no competing interests.
Figures
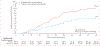

Comment in
-
The therapeutic validation of long COVID.Lancet Infect Dis. 2023 Oct;23(10):1096-1097. doi: 10.1016/S1473-3099(23)00355-9. Epub 2023 Jun 8. Lancet Infect Dis. 2023. PMID: 37302405 Free PMC article. No abstract available.
References
-
- US Centers for Disease Control and Prevention. Post-COVID conditions: information for healthcare providers. 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-c... (accessed Dec 11, 2022).
-
- Cutler DM. The costs of long COVID. JAMA Health Forum 2022; 3: e221809. - PubMed
-
- US Centers for Disease Control and Prevention. Long COVID. 2022. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed Dec 12, 2022).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K23 HL166783/HL/NHLBI NIH HHS/United States
- K23 DK124654/DK/NIDDK NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- UM1 TR004406/TR/NCATS NIH HHS/United States
- OT2 HL161847/HL/NHLBI NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- U54 CA210190/CA/NCI NIH HHS/United States
- T32 HL129956/HL/NHLBI NIH HHS/United States
- R21 LM012744/LM/NLM NIH HHS/United States
- OT2 HL156812/HL/NHLBI NIH HHS/United States
- R01 LM012982/LM/NLM NIH HHS/United States
- K23 HL133604/HL/NHLBI NIH HHS/United States
- KL2 TR002492/TR/NCATS NIH HHS/United States
- P01 CA254849/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

