Memory T follicular helper cells drive donor-specific antibodies independent of memory B cells and primary germinal center and alloantibody formation
- PMID: 37302575
- PMCID: PMC11228286
- DOI: 10.1016/j.ajt.2023.06.006
Memory T follicular helper cells drive donor-specific antibodies independent of memory B cells and primary germinal center and alloantibody formation
Abstract
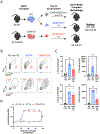
Human leukocyte antigen antibodies are important immunologic mediators of renal allograft loss and are difficult to control. The inability to permanently eliminate donor-specific antibodies (DSA) is partly due to an incomplete understanding of the cellular mechanisms driving alloantibody formation, recurrence, and maintenance. Memory T follicular helper (mTfh) cells rapidly interact with memory B cells upon antigen re-exposure for anamnestic humoral responses, but little is known about Tfh memory in transplantation. We hypothesized that alloreactive mTfh cells form after transplantation and play a critical role in DSA formation following alloantigen re-encounter. To test this hypothesis, we utilized murine skin allograft models to identify and characterize Tfh memory and interrogate its ability to mediate alloantibody responses. We identified alloreactive Tfh memory as a mediator of accelerated humoral alloresponses independent of memory B cells and primary germinal center, or DSA, formation. Furthermore, we demonstrate that mTfh-driven alloantibody formation is susceptible to CD28 costimulation blockade. These findings provide novel insight into a pathologic role for memory Tfh in alloantibody responses and strongly support shifting therapeutic focus from the singular targeting of B cell lineage cells and alloantibodies themselves to multimodal strategies that include inhibition of mTfh cells to treat DSA.
Copyright © 2023 American Society of Transplantation & American Society of Transplant Surgeons. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:I. Raul Badell reports a relationship with Bristol Myers Squibb Co that includes: funding grants. I. Raul Badell reports a relationship with Veloxis Pharmaceuticals Inc that includes: consulting or advisory.
Figures





Similar articles
-
Superior inhibition of alloantibody responses with selective CD28 blockade is CTLA-4 dependent and T follicular helper cell specific.Am J Transplant. 2021 Jan;21(1):73-86. doi: 10.1111/ajt.16004. Epub 2020 Jun 11. Am J Transplant. 2021. PMID: 32406182 Free PMC article.
-
Germinal Center Alloantibody Responses Mediate Progression of Chronic Allograft Injury.Front Immunol. 2019 Jan 23;9:3038. doi: 10.3389/fimmu.2018.03038. eCollection 2018. Front Immunol. 2019. PMID: 30728823 Free PMC article.
-
Circulating T follicular helper cells are a biomarker of humoral alloreactivity and predict donor-specific antibody formation after transplantation.Am J Transplant. 2020 Jan;20(1):75-87. doi: 10.1111/ajt.15517. Epub 2019 Jul 24. Am J Transplant. 2020. PMID: 31250973 Free PMC article.
-
TFH cells in bystander and cognate interactions with B cells.Immunol Rev. 2019 Mar;288(1):28-36. doi: 10.1111/imr.12747. Immunol Rev. 2019. PMID: 30874359 Review.
-
Regulation of Alloantibody Responses.Front Cell Dev Biol. 2021 Jul 8;9:706171. doi: 10.3389/fcell.2021.706171. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34307385 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

