Association of HER2DX with pathological complete response and survival outcomes in HER2-positive breast cancer
- PMID: 37302750
- PMCID: PMC10735273
- DOI: 10.1016/j.annonc.2023.05.012
Association of HER2DX with pathological complete response and survival outcomes in HER2-positive breast cancer
Abstract
Background: The HER2DX genomic test predicts pathological complete response (pCR) and survival outcome in early-stage HER2-positive (HER2+) breast cancer. Here, we evaluated the association of HER2DX scores with (i) pCR according to hormone receptor status and various treatment regimens, and (ii) survival outcome according to pCR status.
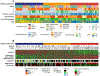
Materials and methods: Seven neoadjuvant cohorts with HER2DX and clinical individual patient data were evaluated (DAPHNe, GOM-HGUGM-2018-05, CALGB-40601, ISPY-2, BiOnHER, NEOHER and PAMELA). All patients were treated with neoadjuvant trastuzumab (n = 765) in combination with pertuzumab (n = 328), lapatinib (n = 187) or without a second anti-HER2 drug (n = 250). Event-free survival (EFS) and overall survival (OS) outcomes were available in a combined series of 268 patients (i.e. NEOHER and PAMELA) with a pCR (n = 118) and without a pCR (n = 150). Cox models were adjusted to evaluate whether HER2DX can identify patients with low or high risk beyond pCR status.
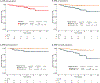
Results: HER2DX pCR score was significantly associated with pCR in all patients [odds ratio (OR) per 10-unit increase = 1.59, 95% confidence interval 1.43-1.77; area under the ROC curve = 0.75], with or without dual HER2 blockade. A statistically significant increase in pCR rate due to dual HER2 blockade over trastuzumab-only was observed in HER2DX pCR-high tumors treated with chemotherapy (OR = 2.36 (1.09-5.42). A statistically significant increase in pCR rate due to multi-agent chemotherapy over a single taxane was observed in HER2DX pCR-medium tumors treated with dual HER2 blockade (OR = 3.11, 1.54-6.49). The pCR rates in HER2DX pCR-low tumors were ≤30.0% regardless of treatment administered. After adjusting by pCR status, patients identified as HER2DX low-risk had better EFS (P < 0.001) and OS (P = 0.006) compared with patients with HER2DX high-risk.
Conclusions: HER2DX pCR score and risk score might help identify ideal candidates to receive neoadjuvant dual HER2 blockade in combination with a single taxane in early-stage HER2+ breast cancer.
Keywords: HER2; HER2DX; breast cancer; neoadjuvant; pathological complete response; pertuzumab.
Copyright © 2023 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest Disclosure
G.V has received a speaker’s fee from MSD, Pfizer, GSK and Pierre Fabrer, has held an advisory role with AstraZeneca and received consultant fees from Reveal Genomics. C. B-M received honoraria as speakers’ honoraria from Roche, Novartis, Lilly, M.S.D and AstraZeneca and G.S.K and Travel Grant from Roche, Novartis and Pfizer. I.E. reports Speaker fees from Roche, Teva, Novartis, Pfizer, Lily and advisory role from Lilly and Astrazeneca. S.L-T reports consulting or advisory role fees from AstraZeneca, Novartis, Roche, Pfizer, Pierre Fabre, Lilly, Seagen, Daichii-Sankyo Europe GmbH, Gilead Sciences, MDS, GlaxoSmithKline, Veracyte and speakers Bureau fees from Lilly. T.M. reports consulting or advisory boards fees from AstraZeneca, Novartis, Roche, GSK and travel grants from Novartis and Astra Zeneca. Y.J. reports consultant or advisory role fees from Novartis, Pfizer, Roche, and AstraZeneca, she received speaker honoraria from Roche, Novartis, Lilly and AstraZeneca, travel and trainnig grants from Roche, Novartis, Pfizer, and Lilly. JA.G-S reports consulting and advisory services fees for Seagen, Gilead, Sanofi. Consultancy/speaker fees from Novartis, Celgene, Eli Lilly, EISAI, AstraZeneca, Daiichi Sankyo, MSD, Pierre Fabre. Institution research funding from AstraZeneca and travel support from Novartis, Roche, Pfizer. F.M. reports consulting or advisory role fees from Roche/Genentech, Novartis, Pfizer, AstraZeneca, MSD, Daiichi Sankyo/Astrazeneca and speakers' bureau fees from Pfizer. Travel, Accommodations, Expenses from Roche/Genentech, Daiichi Sankyo/Astra Zeneca. A.R-L reports consultant Fees from Roche, AstraZeneca, Pfizer, Seagen, Novartis, Lilly, AstraZeneca and Pierre-Fabré and Speaker Bureau fees from Daiichi-Sankyo, Msd and Gilead. AI.B. reports consulting or advisory fees from Pfizer, Novartis, Seagen, Roche, Pierre Fabre. Travel and accommodations fees from Pfizer. A.P. reports advisory and consulting fees from Roche, Pfizer, Novartis, Amgen, BMS, Puma, Oncolytics Biotech, MSD, Guardant Health, Peptomyc and Lilly, lecture fees from Roche, Pfizer, Novartis, Amgen, BMS, Nanostring Technologies and Daiichi Sankyo, institutional financial interests from Boehringer, Novartis, Roche, Nanostring, Sysmex Europa GmbH, Medica Scientia Innovation Research, SL, Celgene, Astellas and Pfizer; stockholder and consultant of Reveal Genomics, SL; A.P. is also listed as an inventor on patent applications for the HER2DX assay. C.M.P is an equity stockholder and consultant of BioClassifier LLC, and for Reveal Genomics. C.M.P is also listed as an inventor on patent applications for the Breast PAM50 assay. J.S.P is an equity stockholder and consultant for Reveal Genomics and is also listed as an inventor on patent applications for the Breast PAM50 assay. F.B-M. has a patent application EP21383165. L.P is listed as an inventor on patent PCT/EP2021/070788. F.S. has no competing financial and non-financial interests. EAM reports compensated service on scientific advisory boards for AstraZeneca, Exact sciences (formerly Genomic Health), Merck, Roche/Genentech; uncompensated service on steering committees for Bristol Myers Squibb, Lilly, and Roche/Genentech; and institutional research support from Roche/Genentech (via SU2C grant) and Gilead. She receives research funding from Susan Komen for the Cure for which she serves as a Scientific Advisor. She also reports uncompensated participation as a member of the American Society of Clinical Oncology Board of Directors. SMT has served as a consultant for: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, Odonate Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Gilead, Mersana, Certara, Chugai Pharma, Ellipses Pharma, Infinity, 4D Pharma, OncoSec Medical Inc., BeyondSpring Pharmaceuticals, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics; SMT has received institutional research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, NanoString Technologies, Cyclacel, Nektar, Gilead, Odonate Therapeutics, Sanofi, Seattle Genetics. M.M. reports Research grants from PUMA; consulting/advisory fees from Roche, Novartis, AstraZeneca, Daiichi-Sankyo, Seagen, Lilly, Sanofi; speakers’ honoraria from Seagen, Lilly, AstraZeneca, Pfizer, Daiichi-Sankyo, Roche. Leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid: Chairman, GEICAM Member of Board of Directors, TRIO. The rest of authors declare no conflict of interest. AGW receives institutional research funding from Genentech/Roche, Macrogenics, and Merck.
Figures


References
-
- Cardoso F, Kyriakides S, Ohno S, et al.: Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology 30:1194–1220, 2019 - PubMed
-
- Carey LA, Berry DA, Cirrincione CT, et al.: Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib. Journal of Clinical Oncology 34:542–549, 2016 - PMC - PubMed
-
- Gianni L, Eiermann W, Semiglazov V, et al.: Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. The Lancet 375:377–384, 2010 - PubMed
-
- Gianni L, Pienkowski T, Im Y-H, et al.: Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. The Lancet Oncology 13:25–32, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

