Comparison of the different anti-CD16 antibody clones in the activation and expansion of peripheral blood NK cells
- PMID: 37302991
- PMCID: PMC10258201
- DOI: 10.1038/s41598-023-36200-6
Comparison of the different anti-CD16 antibody clones in the activation and expansion of peripheral blood NK cells
Abstract
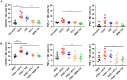
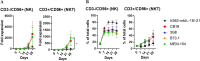
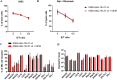
Natural killer (NK) cells are promising tool for cancer treatment. Methods have been developed for large-scale NK cell expansion, including feeder cell-based methods or methods involving stimulation with NK cell activating signals, such as anti-CD16 antibodies. Different clones of anti-CD16 antibodies are available; however, a comprehensive comparison of their differential effects on inducing NK cell activation and expansion has not been conducted among these various clones under the same experimental conditions. Herein, we found that the NK cell expansion rate differed depending on the various anti-CD16 antibodies (CB16, 3G8, B73.1, and MEM-154) coated on microbeads when stimulated with genetically engineered feeder cells, K562‑membrane-bound IL‑18, and mbIL‑21 (K562‑mbIL‑18/-21). Only the CB16 clone combination caused enhanced NK cell expansion over K562‑mbIL‑18/-21 stimulation alone with similar NK cell functionality. Treatment with the CB16 clone once on the initial day of NK cell expansion was sufficient to maximize the combination effect. Overall, we developed a more enhanced NK expansion system by merging a feeder to effectively stimulate CD16 with the CB16 clone.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

