Efficacy and Safety of Eculizumab in Pediatric Patients Affected by Shiga Toxin-Related Hemolytic and Uremic Syndrome: A Randomized, Placebo-Controlled Trial
- PMID: 37303085
- PMCID: PMC10482062
- DOI: 10.1681/ASN.0000000000000182
Efficacy and Safety of Eculizumab in Pediatric Patients Affected by Shiga Toxin-Related Hemolytic and Uremic Syndrome: A Randomized, Placebo-Controlled Trial
Abstract
Significance statement: Shiga toxin-related hemolytic uremic syndrome (STEC-HUS) is a serious condition, characterized by multiorgan thrombotic microangiopathy, mainly affecting children. Renal involvement is severe, with approximately half of patients requiring dialysis. So far, no specific treatment has been proven efficient in STEC-HUS. The use of eculizumab, a monoclonal antibody inhibiting terminal complement complex, has demonstrated remarkable success in atypical hemolytic uremic syndrome, but its use in uncontrolled studies to treat STEC-HUS has yielded inconsistent results. In this Phase 3 randomized, placebo-controlled trial in 100 pediatric patients with STEC-HUS, the findings did not show efficacy of eculizumab during the acute phase of the disease. However, the results indicated a reduction of renal sequelae in eculizumab-treated patients at 1-year follow-up. Larger prospective studies would be needed to further explore eculizumab as a potential treatment.
Background: Shiga toxin-related hemolytic uremic syndrome (STEC-HUS) in children is a severe condition, resulting in approximately 50% of patients requiring RRT. Furthermore, at least 30% of survivors experience kidney sequelae. Recently, activation of the complement alternative pathway has been postulated as a factor in STEC-HUS pathophysiology, leading to compassionate use of eculizumab, a monoclonal antibody inhibiting the terminal complement complex, in affected patients. Given the lack of therapy for STEC-HUS, a controlled study of eculizumab efficacy in treating this condition is a priority.
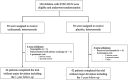
Methods: We conducted a Phase 3 randomized trial of eculizumab in children with STEC-HUS. Patients were randomly assigned in a 1:1 ratio to receive either eculizumab or placebo during 4 weeks. Follow-up lasted for 1 year. The primary end point was RRT duration <48 hours after randomization. Secondary endpoints included hematologic and extrarenal involvement.
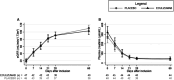
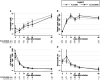
Results: Baseline characteristics were similar among the 100 patients who underwent randomization. The rate of RRT <48 hours did not differ significantly between the two groups (48% in the placebo versus 38% in the eculizumab group; P = 0.31) or in the course of ARF. The two groups also exhibited similar hematologic evolution and extrarenal manifestations of STEC-HUS. The proportion of patients experiencing renal sequelae at 1 year was lower in the eculizumab group than in the placebo group (43.48% and 64.44%, respectively, P = 0.04). No safety concern was reported.
Conclusions: In pediatric patients with STEC-HUS, eculizumab treatment does not appear to be associated with improved renal outcome during acute phase of the disease but may reduce long-term kidney sequelae.
Clinical trials registrations: EUDRACT (2014-001169-28) ClinicalTrials.gov ( NCT02205541 ).
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
C. Brusq reports Consultancy: Medtronic France SAS; Honoraria: Laboratoires Grünenthal SAS, Lundbeck SAS, and Medtronic France SAS; and Speakers Bureau: Laboratoires Grünenthal SAS, Lundbeck SAS, and Medtronic France SAS. M. Fila reports Consultancy: Alexion and Roche; and Honoraria: Alexion. V. Frémeaux-Bacchi reports Consultancy: Alexion Pharmaceuticals, Apellis, BioCryps, Novartis, Roche, Sobi, and UCB; Research Funding: Alexion Pharmaceuticals; Honoraria: Alexion Pharmaceuticals, Apellis, BioCryps, Novartis, Roche, Sobi, and UCB; and Advisory or Leadership Role: Alexion Pharmaceuticals, Apellis, BioCryps, Novartis, and Sobi. V. Guigonis reports Research Funding: INRESA. J. Harambat reports Consultancy: Alnylam; Research Funding: Alnylam, Bayer, and GSK; Honoraria: Alnylam; Advisory or Leadership Role: ESPN Council Member, ESPN/ERA-EDTA Chairman, and
Figures



Comment in
-
Eculizumab Seems to be a Life Saver Even in Shiga Toxin-Related Hemolytic-Uremic Syndrome.J Am Soc Nephrol. 2024 Mar 1;35(3):384. doi: 10.1681/ASN.0000000000000283. J Am Soc Nephrol. 2024. PMID: 38427451 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

