Selective pharmacological inhibition alters human carcinoma lung cell-derived extracellular vesicle formation
- PMID: 37303541
- PMCID: PMC10250759
- DOI: 10.1016/j.heliyon.2023.e16655
Selective pharmacological inhibition alters human carcinoma lung cell-derived extracellular vesicle formation
Abstract
Exosomes also termed small extracellular vesicles (sEVs) are important mediators of intercellular communication in many physiological and pathological processes such as protein clearance, immunity, infections, signaling, and cancer. Elevated circulating levels of exosomes have been linked to some viral infections, aggressive cancer, and neurodegenerative diseases. Some pharmacological compounds have been demonstrated to effectively inhibit exosome production pathways. There are very few studies on exosome inhibition and how they influence pathophysiological conditions.
Methods: In the current study, we examined how inhibition of extracellular vesicle release and/or uptake would impact the exosome formation pathway. Using a constellation of improved EV experimental approaches, we evaluated the concentration-based cytotoxicity effects of pharmacological agents (ketoconazole, climbazole, and heparin) on Human Lung Carcinoma (A549) cell viability. We investigated the effect of inhibitor dosages on exosome production and release. Analysis of exosome inhibition includes quantitative analysis and total protein expression of exosome release after pharmacological inhibition; we examined exosome protein level after inhibition.
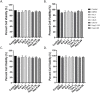
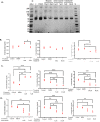
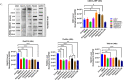
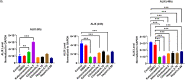
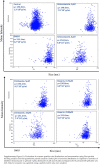
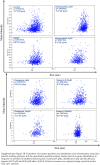
Results: Selective inhibition of exosomes altered particle sizes, and heparin significantly reduced the total exosomes released. Climbazole and heparin undermined membrane-bound tetraspanin CD63 expression and significantly disrupted ALIX protein (p ≤ 0.0001) and TSG101 (p ≤ 0.001). Azoles and heparin also disrupt transmembrane trafficking by modulating Ras binding protein (p ≤ 0.001).
Conclusion: These findings revealed that pharmacological inhibition of exosomes regulates the endocytic pathway and expression of endosomal sorting complex required for transport mediators, suggesting climbazole and heparin as effective inhibitors of exosome synthesis.
Keywords: ESCRT; Extracellular vesicles; Inhibition; Pathway.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures









References
LinkOut - more resources
Full Text Sources
Miscellaneous

