Effects of SMOF on soil properties, root-zone microbial community structure, metabolites, and maize (Zea mays L.) response on a reclaimed barren mountainous land
- PMID: 37303787
- PMCID: PMC10248427
- DOI: 10.3389/fmicb.2023.1181245
Effects of SMOF on soil properties, root-zone microbial community structure, metabolites, and maize (Zea mays L.) response on a reclaimed barren mountainous land
Abstract
Introduction: Maize is the largest crop produced in China. With the growing population and the rapid development of urbanization and industrialization, maize has been recently cultivated in reclaimed barren mountainous lands in Zhejiang Province, China. However, the soil is usually not suitable for cultivation because of its low pH and poor nutrient conditions. To improve soil quality for crop growth, various fertilizers, including inorganic, organic, and microbial fertilizers, were used in the field. Among them, organic fertilizer-based sheep manure greatly improved the soil quality and has been widely adopted in reclaimed barren mountainous lands. But the mechanism of action was not well clear.
Methods: The field experiment (SMOF, COF, CCF and the control) was carried out on a reclaimed barren mountainous land in Dayang Village, Hangzhou City, Zhejiang Province, China. To systematically evaluate the effect of SMOF on reclaimed barren mountainous lands, soil properties, the root-zone microbial community structure, metabolites, and maize response were investigated.
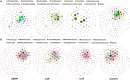
Results: Compared with the control, SMOF could not significantly affect the soil pH but caused 46.10%, 28.28%, 101.94%, 56.35%, 79.07%, and 76.07% increases in the OMC, total N, available P, available K, MBC, and MBN, respectively. Based on 16S amplicon sequencing of soil bacteria, compared with the control, SMOF caused a 11.06-334.85% increase in the RA of Ohtaekwangia, Sphingomonas, unclassified_Sphingomonadaceae, and Saccharibacteria and a 11.91-38.60% reduction in the RA of Spartobacteria, Gemmatimonas, Gp4, Flavisolibacter, Subdivision3, Gp6, and unclassified_Betaproteobacteria, respectively. Moreover, based on ITS amplicon sequencing of soil fungi, SMOF also caused a 42.52-330.86% increase in the RA of Podospora, Clitopilus, Ascobolus, Mortierella, and Sordaria and a 20.98-64.46% reduction in the RA of Knufia, Fusarium, Verticillium, and Gibberella, respectively, compared with the control. RDA of microbial communities and soil properties revealed that the main variables of bacterial and fungal communities included available K, OMC, available P, MBN, and available K, pH, and MBC, respectively. In addition, LC-MS analysis indicated that 15 significant DEMs belonged to benzenoids, lipids, organoheterocyclic compounds, organic acids, phenylpropanoids, polyketides, and organic nitrogen compounds in SMOF and the control group, among which four DEMs were significantly correlated with two genera of bacteria and 10 DEMs were significantly correlated with five genera of fungi. The results revealed complicated interactions between microbes and DEMs in the soil of the maize root zone. Furthermore, the results of field experiments demonstrated that SMOF could cause a significant increase in maize ears and plant biomass.
Conclusions: Overall, the results of this study showed that the application of SMOF not only significantly modified the physical, chemical, and biological properties of reclaimed barren mountainous land but also promoted maize growth. SMOF can be used as a good amendment for maize production in reclaimed barren mountainous lands.
Keywords: SMOF; maize; metabonomics; microbial community; reclaimed barren mountainous land; soil property.
Copyright © 2023 Li, Wang, Lu, Tian and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











References
-
- Alhrout H. H., Akash M. W., Hejazin R. K. (2018). Effect of farm yard manure and NPK on the yield and some growth components of tomato (Lycopersicum esculentum). Res. Crops 19, 655–658. 10.31830/2348-7542.2018.0001.43 - DOI
-
- Amanullah I., Hidayatullah K., Jan A., Shah Z., Jamal Khan M., Parmar B., et al. . (2019). “Organic carbon sources and nitrogen management improve biomass of hybrid rice (Oryza sativa L.) under nitrogen deficient condition,” in Advances in Rice Research for Abiotic Stress Tolerance, eds M. Hasanuzzaman, M. Fujita, and K. Nahar (Amsterdam: Elsevier; ), 447–467. 10.1016/B978-0-12-814332-2.00022-8 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

