Long noncoding RNA PPP1R14B-AS1 imitates microRNA-134-3p to facilitate breast cancer progression by upregulating LIM and SH3 protein 1
- PMID: 37303940
- PMCID: PMC10207964
- DOI: 10.32604/or.2022.03582
Long noncoding RNA PPP1R14B-AS1 imitates microRNA-134-3p to facilitate breast cancer progression by upregulating LIM and SH3 protein 1
Abstract
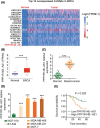
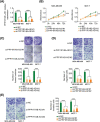
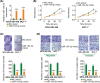
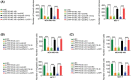
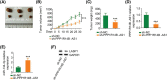
Long noncoding RNA PPP1R14B antisense RNA 1 (PPP1R14B-AS1) has emerged as a critical modulator of liver cancer and lung adenocarcinoma progression. However, the functional importance and biological relevance of PPP1R14B-AS1 in breast cancer remain unclear. Therefore, this study was designed to detect PPP1R14B-AS1 levels in breast cancer cells using qRT-PCR and elucidate the influence of PPP1R14B-AS1 on aggressive phenotypes. Furthermore, molecular events mediating the action of PPP1R14B-AS1 were characterized in detail. Functional experiments addressed the impacts of PPP1R14B-AS1 knockdown on breast cancer cells. In this study, PPP1R14B-AS1 was found to be overexpressed in breast cancer, exhibiting a close correlation with poor patient prognosis. Results also showed that breast cancer cell proliferation and motility were suppressed when PPP1R14B-AS1 was silenced. Mechanistically, PPP1R14B-AS1 acted as a competing endogenous RNA for microRNA-134-3p (miR-134-3p) in breast cancer cells. PPP1R14B-AS1 also increased LIM and SH3 protein 1 (LASP1) levels by imitating miR-134-3p in breast cancer cells. Rescue experiments further corroborated that the knockdown of miR-134-3p or an increase in LASP1 restored the aggressive malignant characteristics of breast cancer cells that were weakened by PPP1R14B-AS1 depletion. In summary, PPP1R14B-AS1 facilitated the oncogenicity of breast cancer cells by controlling the miR-134-3p/LASP1 axis. We believe that our findings may contribute to the development of precision therapy techniques in the field of breast cancer treatment.
Keywords: Precision therapy; Therapeutic target; ceRNA theory; miRNA sponge.
© 2022 Zhou et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest to report regarding the present study.
Figures




Similar articles
-
LncRNA NNT-AS1 promotes non-small cell lung cancer progression through regulating miR-22-3p/YAP1 axis.Thorac Cancer. 2020 Mar;11(3):549-560. doi: 10.1111/1759-7714.13280. Epub 2020 Jan 10. Thorac Cancer. 2020. PMID: 31923353 Free PMC article.
-
LncRNA CBR3-AS1 predicts a poor prognosis and promotes cervical cancer progression through the miR-3163/LASP1 pathway.Neoplasma. 2022 Dec;69(6):1406-1417. doi: 10.4149/neo_2022_220730N784. Neoplasma. 2022. PMID: 36591804
-
CERS6-AS1 Facilitates Oncogenesis and Restrains Ferroptosis in Papillary Thyroid Carcinoma by Serving as a ceRNA through miR-497-5p/LASP1 Axis.Ann Clin Lab Sci. 2022 May;52(3):426-438. Ann Clin Lab Sci. 2022. PMID: 35777805
-
Long noncoding RNA TFAP2A-AS1 exerts promotive effects in non-small cell lung cancer progression via controlling the microRNA-548a-3p/CDK4 axis as a competitive endogenous RNA.Oncol Res. 2022 Jul 13;29(2):129-139. doi: 10.32604/or.2022.03563. eCollection 2021. Oncol Res. 2022. PMID: 37305398 Free PMC article.
-
Circ_0023028 contributes to the progression of laryngeal squamous cell carcinoma by upregulating LASP1 through miR-486-3p.Mol Cell Biochem. 2021 Aug;476(8):2951-2961. doi: 10.1007/s11010-021-04129-x. Epub 2021 Mar 23. Mol Cell Biochem. 2021. PMID: 33755879
Cited by
-
lncRNA PAARH impacts liver cancer cell proliferation by engaging miR‑6512‑3p to target LASP1.Oncol Lett. 2024 May 8;28(1):306. doi: 10.3892/ol.2024.14439. eCollection 2024 Jul. Oncol Lett. 2024. PMID: 38774456 Free PMC article.
-
The emerging role of miRNAs in biological aging and age-related diseases.Noncoding RNA Res. 2025 May 5;13:131-152. doi: 10.1016/j.ncrna.2025.05.002. eCollection 2025 Aug. Noncoding RNA Res. 2025. PMID: 40501482 Free PMC article. Review.
-
PPP1R14B as a potential biomarker for the identification of diagnosis and prognosis affecting tumor immunity, proliferation and migration in prostate cancer.J Cancer. 2024 Oct 21;15(20):6545-6564. doi: 10.7150/jca.101100. eCollection 2024. J Cancer. 2024. PMID: 39668827 Free PMC article.
-
Rediscovery of PHI-1/PPP1R14B: Emerging Roles of Cellular PP1 Signaling Mediated by the PPP1R14B Gene Product in Multiple Cancers and Beyond.Biomolecules. 2025 Feb 27;15(3):344. doi: 10.3390/biom15030344. Biomolecules. 2025. PMID: 40149880 Free PMC article. Review.
References
-
- Wormann, B. (2017). Breast cancer: Basics, screening, diagnostics and treatment. Medizinische Monatsschrift fur Pharmazeuten , 40(2), 55–64. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
