PATHPOD - A loop-mediated isothermal amplification (LAMP)-based point-of-care system for rapid clinical detection of SARS-CoV-2 in hospitals in Denmark
- PMID: 37304211
- PMCID: PMC10245468
- DOI: 10.1016/j.snb.2023.134085
PATHPOD - A loop-mediated isothermal amplification (LAMP)-based point-of-care system for rapid clinical detection of SARS-CoV-2 in hospitals in Denmark
Abstract
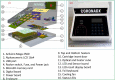
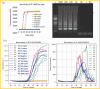
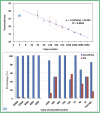
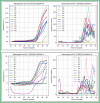
Sensitive and rapid detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a vital goal in the ongoing COVID-19 pandemic. We present in this comprehensive work, for the first time, detailed fabrication and clinical validation of a point of care (PoC) device for rapid, onsite detection of SARS-CoV-2 using a real-time reverse-transcription loop-mediated isothermal amplification (RT-LAMP) reaction on a polymer cartridge. The PoC system, namely PATHPOD, consisting of a standalone device (weight less than 1.2 kg) and a cartridge, can perform the detection of 10 different samples and two controls in less than 50 min, which is much more rapid than the golden standard real-time reverse-transcription Polymerase Chain Reaction (RT-PCR), typically taking 16-48 h. The novel total internal reflection (TIR) scheme and the reactions inside the cartridge in the PoC device allow monitoring of the diagnostic results in real-time and onsite. The analytical sensitivity and specificity of the PoC test are comparable with the current RT-PCR, with a limit of detection (LOD) down to 30-50 viral genome copies. The robustness of the PATHPOD PoC system has been confirmed by analyzing 398 clinical samples initially examined in two hospitals in Denmark. The clinical sensitivity and specificity of these tests are discussed.
Keywords: COVID-19 pandemic; Clinical sensitivity; Limit of detection (LOD); Point of care (PoC) device; Reverse-transcription, loop-mediated isothermal amplification; SARS-CoV-2.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
A Multiplex and Colorimetric Reverse Transcription Loop-Mediated Isothermal Amplification Assay for Sensitive and Rapid Detection of Novel SARS-CoV-2.Front Cell Infect Microbiol. 2021 Jun 29;11:653616. doi: 10.3389/fcimb.2021.653616. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34268131 Free PMC article.
-
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies.Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668736 Free PMC article.
-
Current state of diagnostic, screening and surveillance testing methods for COVID-19 from an analytical chemistry point of view.Microchem J. 2021 Aug;167:106305. doi: 10.1016/j.microc.2021.106305. Epub 2021 Apr 19. Microchem J. 2021. PMID: 33897053 Free PMC article.
-
Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Sensitive, Specific, and Cost-Effective Point-of-Care Test for Coronaviruses in the Context of COVID-19 Pandemic.Biology (Basel). 2020 Jul 22;9(8):182. doi: 10.3390/biology9080182. Biology (Basel). 2020. PMID: 32707972 Free PMC article. Review.
-
A Novel Real-Time Reverse Transcription Loop-Mediated Isothermal Amplification Detection Platform: Application to Diagnosis of COVID-19.Front Bioeng Biotechnol. 2021 Oct 22;9:748746. doi: 10.3389/fbioe.2021.748746. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34746104 Free PMC article. Review.
Cited by
-
Development of Loop-Mediated Isothermal Amplification Method for Rapid and Sensitive Identification of Hermetia illucens (Diptera: Stratiomyidae).Methods Protoc. 2023 Sep 5;6(5):81. doi: 10.3390/mps6050081. Methods Protoc. 2023. PMID: 37736964 Free PMC article.
-
Advances in Simple, Rapid, and Contamination-Free Instantaneous Nucleic Acid Devices for Pathogen Detection.Biosensors (Basel). 2023 Jul 14;13(7):732. doi: 10.3390/bios13070732. Biosensors (Basel). 2023. PMID: 37504131 Free PMC article. Review.
-
Low-Cost Arduino Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) for Sensitive Nucleic Acid Detection.Biosensors (Basel). 2024 Feb 29;14(3):128. doi: 10.3390/bios14030128. Biosensors (Basel). 2024. PMID: 38534235 Free PMC article.
-
The synergy of nucleic acid amplification and miniaturized systems in enhancing liquid biopsy applications.Bioanalysis. 2024 Jun 2;16(11):499-504. doi: 10.4155/bio-2023-0238. Epub 2024 Feb 21. Bioanalysis. 2024. PMID: 38380670 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
