Results of haploidentical transplant in patients with donor-specific antibodies: a survey on behalf of the Spanish Group of Hematopoietic Transplant and Cell Therapy
- PMID: 37304258
- PMCID: PMC10250708
- DOI: 10.3389/fimmu.2023.1165759
Results of haploidentical transplant in patients with donor-specific antibodies: a survey on behalf of the Spanish Group of Hematopoietic Transplant and Cell Therapy
Abstract
Background: Donor-specific antibodies (DSAs) are IgG allo-antibodies against mismatched donor HLA molecules and can cause graft failure (GF) in the setting of haploidentical hematopoietic stem cell transplantation (haplo-HSCT). Our aim was to report the experience of the Spanish Group of Hematopoietic Transplant (GETH-TC) in DSA-positive patients who had undergone haplo-HSCT.
Methods: We conducted a survey of patients who underwent haplo-HSCT in GETH-TC centers between 2012 and 2021. Data were collected on the DSA assay used, monitoring strategy, complement fixation, criteria for desensitization, desensitization strategies and transplant outcomes.
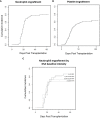
Results: Fifteen centers from the GETH-TC responded to the survey. During the study period, 1,454 patients underwent haplo-HSCT. Seventy of the transplants were performed in 69 DSA-positive patients, all of whom lacked a suitable alternative donor; 61 (88%) patients were female (90% with prior pregnancies). All patients received post-transplant cyclophosphamide-based graft-versus-host disease prophylaxis. Regarding baseline DSA intensity, 46 (67%) patients presented mean fluorescence intensity (MFI) >5,000, including 21 (30%) with MFI >10,000 and three (4%) with MFI >20,000. Six patients did not receive desensitization treatment, four of them with MFI <5,000. Of 63 patients receiving desensitization treatment, 48 (76%) were tested after desensitization therapy, and a reduction in intensity was confirmed in 45 (71%). Three patients (5%) experienced an increase in MFI after desensitization, two of whom experienced primary GF. Cumulative incidence of neutrophil engraftment at day 28 was 74% in a median of 18 days (IQR, 15─20); six patients died before engraftment due to toxicity or infection and eight patients had primary GF despite desensitization in seven of them. After a median follow-up of 30 months, two-year overall and event-free survival were 46.5% and 39%, respectively. The two-year cumulative incidence of relapse was 16% and non-relapse mortality (NRM) was 43%. Infection was the most frequent cause of NRM, followed by endothelial toxicity. Multivariate analysis identified baseline MFI >20,000 as an independent risk factor for survival and an increase in titers after infusion as an independent risk factor for GF.
Conclusions: Haplo-HSCT is feasible in DSA-positive patients, with high rates of engraftment after desensitization guided by DSA intensity. Baseline MFI >20,000 and increased intensity after infusion are risk factors for survival and GF.
Keywords: DSA kinetics; desensitization strategies; donor-specific antibodies; graft failure; haplo-HSCT.
Copyright © 2023 Bailén, Alenda, Herruzo-Delgado, Acosta-Fleitas, Vallés, Esquirol, Fonseca, Solán, Sánchez-Vadillo, Bautista, Bento, López-Godino, Pérez-Martínez, Torrent, Zanabili, Calbacho, Moreno, Pascual-Cascón, Guerra-Domínguez, Chinea, García-Cadenas, López-Corral, Boix-Giner, López Lorenzo, Humala, Duarte, Sampol, Heras, Vicario, Balas, Oarbeascoa, Fernández-Caldas, Anguita and Kwon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. . Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant (2022) 57(8):1217–1239. doi: 10.1038/s41409-022-01691-w - DOI - PMC - PubMed
-
- Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides, (2021).
-
- Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. . The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood (2010) 115:5. doi: 10.1182/blood-2009-09-244525 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

