Harnessing type I interferon-mediated immunity to target malignant brain tumors
- PMID: 37304294
- PMCID: PMC10247981
- DOI: 10.3389/fimmu.2023.1203929
Harnessing type I interferon-mediated immunity to target malignant brain tumors
Abstract
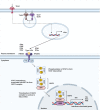
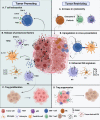
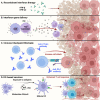
Type I interferons have long been appreciated as a cytokine family that regulates antiviral immunity. Recently, their role in eliciting antitumor immune responses has gained increasing attention. Within the immunosuppressive tumor microenvironment (TME), interferons stimulate tumor-infiltrating lymphocytes to promote immune clearance and essentially reshape a "cold" TME into an immune-activating "hot" TME. In this review, we focus on gliomas, with an emphasis on malignant glioblastoma, as these brain tumors possess a highly invasive and heterogenous brain TME. We address how type I interferons regulate antitumor immune responses against malignant gliomas and reshape the overall immune landscape of the brain TME. Furthermore, we discuss how these findings can translate into future immunotherapies targeting brain tumors in general.
Keywords: brain tumors; glioblastoma; immunotherapy; tumor microenvironment; type I interferons.
Copyright © 2023 Lim, Kang, La, Ku, Kang, Kim, Park and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Immune Infiltrating Cells-Derived Risk Signature Based on Large-scale Analysis Defines Immune Landscape and Predicts Immunotherapy Responses in Glioma Tumor Microenvironment.Front Immunol. 2021 Aug 13;12:691811. doi: 10.3389/fimmu.2021.691811. eCollection 2021. Front Immunol. 2021. PMID: 34489938 Free PMC article.
-
Advances in research on immune escape mechanism of glioma.CNS Neurosci Ther. 2023 Jul;29(7):1709-1720. doi: 10.1111/cns.14217. Epub 2023 Apr 23. CNS Neurosci Ther. 2023. PMID: 37088950 Free PMC article. Review.
-
Fyn tyrosine kinase, a downstream target of receptor tyrosine kinases, modulates antiglioma immune responses.Neuro Oncol. 2020 Jun 9;22(6):806-818. doi: 10.1093/neuonc/noaa006. Neuro Oncol. 2020. PMID: 31950181 Free PMC article.
-
Glioma-associated microglia/macrophages (GAMs) in glioblastoma: Immune function in the tumor microenvironment and implications for immunotherapy.Front Immunol. 2023 Mar 9;14:1123853. doi: 10.3389/fimmu.2023.1123853. eCollection 2023. Front Immunol. 2023. PMID: 36969167 Free PMC article. Review.
-
Dendritic cell vaccine of gliomas: challenges from bench to bed.Front Immunol. 2023 Sep 14;14:1259562. doi: 10.3389/fimmu.2023.1259562. eCollection 2023. Front Immunol. 2023. PMID: 37781367 Free PMC article. Review.
Cited by
-
Impact of developmental state, p53 status, and interferon signaling on glioblastoma cell response to radiation and temozolomide treatment.PLoS One. 2025 Feb 7;20(2):e0315171. doi: 10.1371/journal.pone.0315171. eCollection 2025. PLoS One. 2025. PMID: 39919036 Free PMC article.
-
Combined PARP14 inhibition and PD-1 blockade promotes cytotoxic T cell quiescence and modulates macrophage polarization in relapsed melanoma.J Immunother Cancer. 2025 Jan 27;13(1):e010683. doi: 10.1136/jitc-2024-010683. J Immunother Cancer. 2025. PMID: 39870492 Free PMC article.
-
An Update on the Clinical Status, Challenges, and Future Directions of Oncolytic Virotherapy for Malignant Gliomas.Curr Treat Options Oncol. 2024 Jul;25(7):952-991. doi: 10.1007/s11864-024-01211-6. Epub 2024 Jun 19. Curr Treat Options Oncol. 2024. PMID: 38896326 Free PMC article. Review.
-
Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment.Mol Cancer. 2023 Oct 13;22(1):170. doi: 10.1186/s12943-023-01876-x. Mol Cancer. 2023. PMID: 37833788 Free PMC article.
References
-
- Shekarian T, Zinner CP, Bartoszek EM, Duchemin W, Wachnowicz AT, Hogan S, et al. . Immunotherapy of glioblastoma explants induces interferon-γ; responses and spatial immune cell rearrangements in tumor center, but not periphery. Sci Adv (2022) 8(26):eabn9440. doi: 10.1126/sciadv.abn9440 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

