Single point mutations at the S129 residue of α-synuclein and their effect on structure, aggregation, and neurotoxicity
- PMID: 37304685
- PMCID: PMC10250651
- DOI: 10.3389/fchem.2023.1145877
Single point mutations at the S129 residue of α-synuclein and their effect on structure, aggregation, and neurotoxicity
Abstract
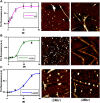
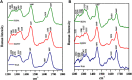
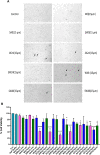
Parkinson's disease is an age-related neurological disorder, and the pathology of the disease is linked to different types of aggregates of α-synuclein or alpha-synuclein (aS), which is an intrinsically disordered protein. The C-terminal domain (residues 96-140) of the protein is highly fluctuating and possesses random/disordered coil conformation. Thus, the region plays a significant role in the protein's solubility and stability by an interaction with other parts of the protein. In the current investigation, we examined the structure and aggregation behavior of two artificial single point mutations at a C-terminal residue at position 129 that represent a serine residue in the wild-type human aS (wt aS). Circular Dichroism (CD) and Raman spectroscopy were performed to analyse the secondary structure of the mutated proteins and compare it to the wt aS. Thioflavin T assay and atomic force microscopy imaging helped in understanding the aggregation kinetics and type of aggregates formed. Finally, the cytotoxicity assay gave an idea about the toxicity of the aggregates formed at different stages of incubation due to mutations. Compared to wt aS, the mutants S129A and S129W imparted structural stability and showed enhanced propensity toward the α-helical secondary structure. CD analysis showed proclivity of the mutant proteins toward α-helical conformation. The enhancement of α-helical propensity lengthened the lag phase of fibril formation. The growth rate of β-sheet-rich fibrillation was also reduced. Cytotoxicity tests on SH-SY5Y neuronal cell lines established that the S129A and S129W mutants and their aggregates were potentially less toxic than wt aS. The average survivability rate was ∼40% for cells treated with oligomers (presumably formed after 24 h of incubation of the freshly prepared monomeric protein solution) produced from wt aS and ∼80% for cells treated with oligomers obtained from mutant proteins. The relative structural stability with α-helical propensity of the mutants could be a plausible reason for their slow rate of oligomerization and fibrillation, and this was also the possible reason for reduced toxicity to neuronal cells.
Keywords: Raman; alpha-synuclein; amyloid; fibrillization; neurotoxicity; secondary structure.
Copyright © 2023 Pandit, Das, Das, Dolui, Saha, Pal, Mondal, Chowdhury, Biswas and Maiti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Anderson J. P., Walker D. E., Goldstein J. M., Laat R., Banducci K., Caccavello R. J., et al. (2006). Phosphorylation of ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease *. J. Biol. Chem. 281, 29739–29752. 10.1074/jbc.M600933200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

